34 b2 molecular orbital diagram
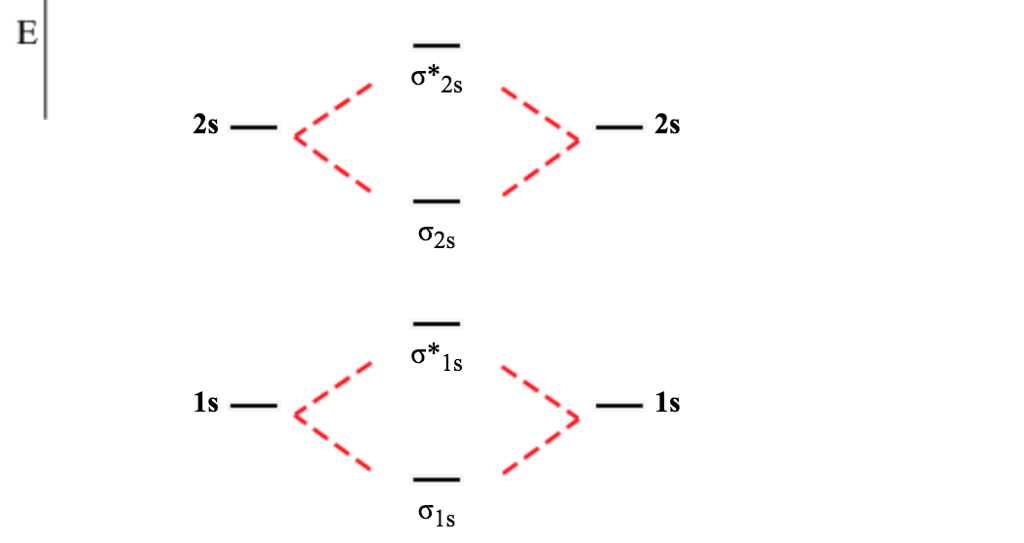
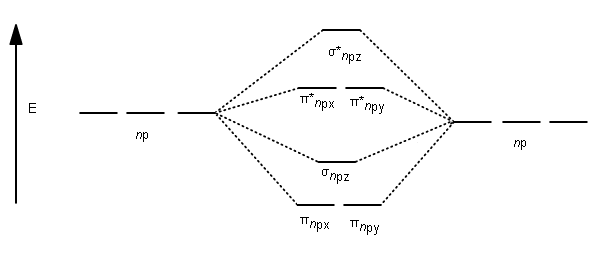
Before we get there it is worth while knowing a generic valence molecular orbital diagram where no s-p mixing occurs. This one pretty much applies to all main group elements heavier than nitrogen. The core orbitals, in case of lithium to neon these are the 1s orbitals, sodium to argon these are 1s, 2s, and 2p orbitals, are not included, as they ... 6:08This video discusses how to draw the molecular orbital (MO) diagram for the B2 (boron) molecule. The bond ...30 May 2021 · Uploaded by Principia
6:25This video discusses how to draw the molecular orbital (MO) diagram for the B2(+) molecule. The bond ...4 Jun 2021 · Uploaded by Principia

B2 molecular orbital diagram
10:50From the periodic table as we have already discussed the Molecular orbital diagrams of diatomic molecules of ...24 Jun 2020 · Uploaded by Edmerls 23.10.2021 · Summary of licorice components properties: molecular weight (MW), number of H-bond donor/acceptor (HA/HD), water solubility (WS), and quantum chemical parameters, including the energy levels of the highest occupied molecular orbital (E HOMO), lowest unoccupied molecular orbital (E LUMO), energy gap (ΔE), dipole moment (μ), electronegativity (χ), global hardness (η) and the … 6:08This video discusses how to draw the molecular orbital (MO) diagram for the B2(-) molecule. The bond order ...2 Jun 2021 · Uploaded by Principia
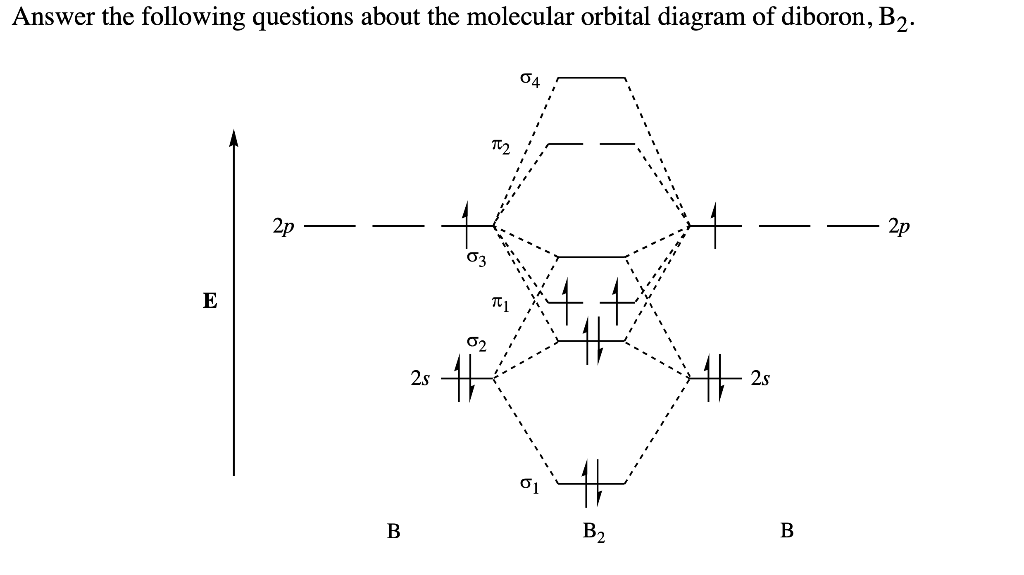
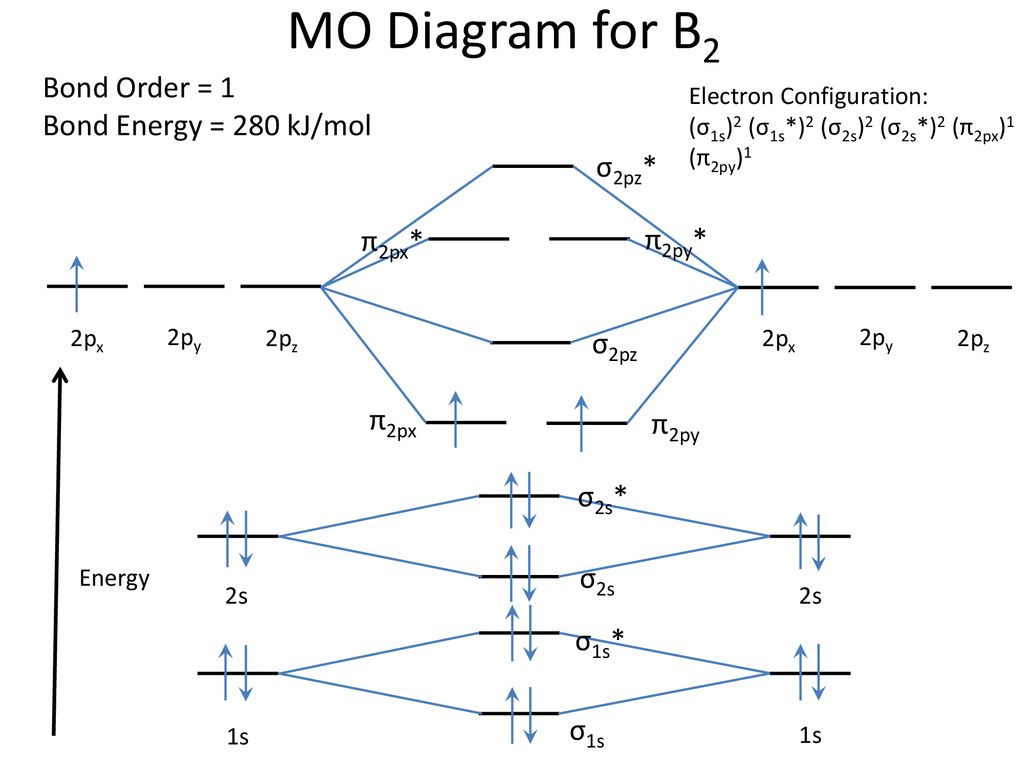
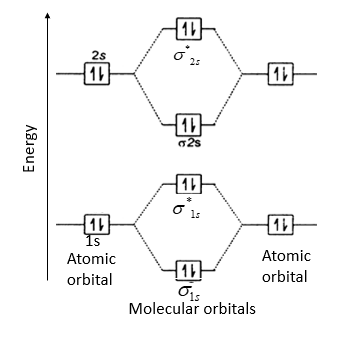
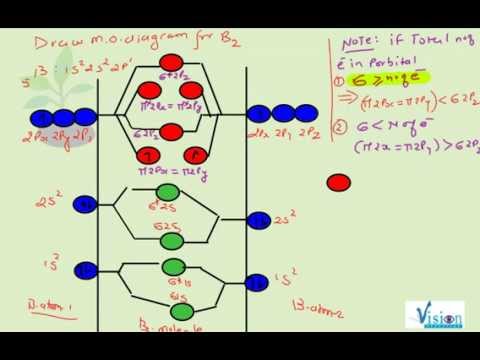
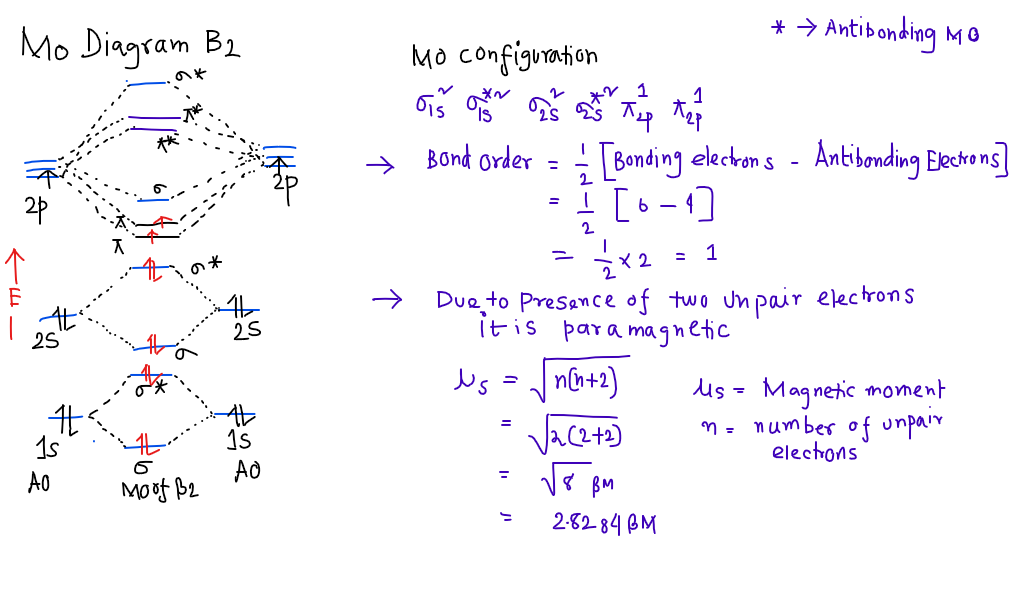
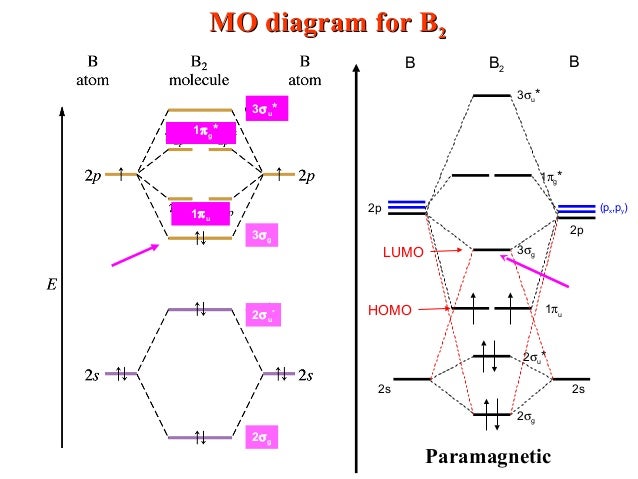
B2 molecular orbital diagram. Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals. The 2s orbitals will overlap to form 2sσ and 2sσ ... Molecular Orbital Theory allows us to predict the distribution of electrons within a molecule. This allows us to predict properties such as bond order, magnetism, and shape . There are two types of MO diagrams: Recall that the bonding MOs are those without an asterisk (e.g., σ1s), while the antibonding MOs are those with an asterisk (e.g., σ1s*). Question: Draw the molecular orbital diagram for B2+ (this is not B-B. it has an electron missing, so its B-B cation!) The number of unpaired electrons in the B2+ molecule is 2 (two) 1 (one) 3 (three) zero . This problem has been solved! See the answer See the answer See the answer done loading. 02.11.2021 · Molecular orbital energy diagram of b2. a. The next two would fill the 1 sigma e antibonding orbital. Individual atomic orbitals ao are arranged on the far left and far right of the diagram. As well, i filled in the sigma 2px and pi 2py orbits. Y: Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals ...
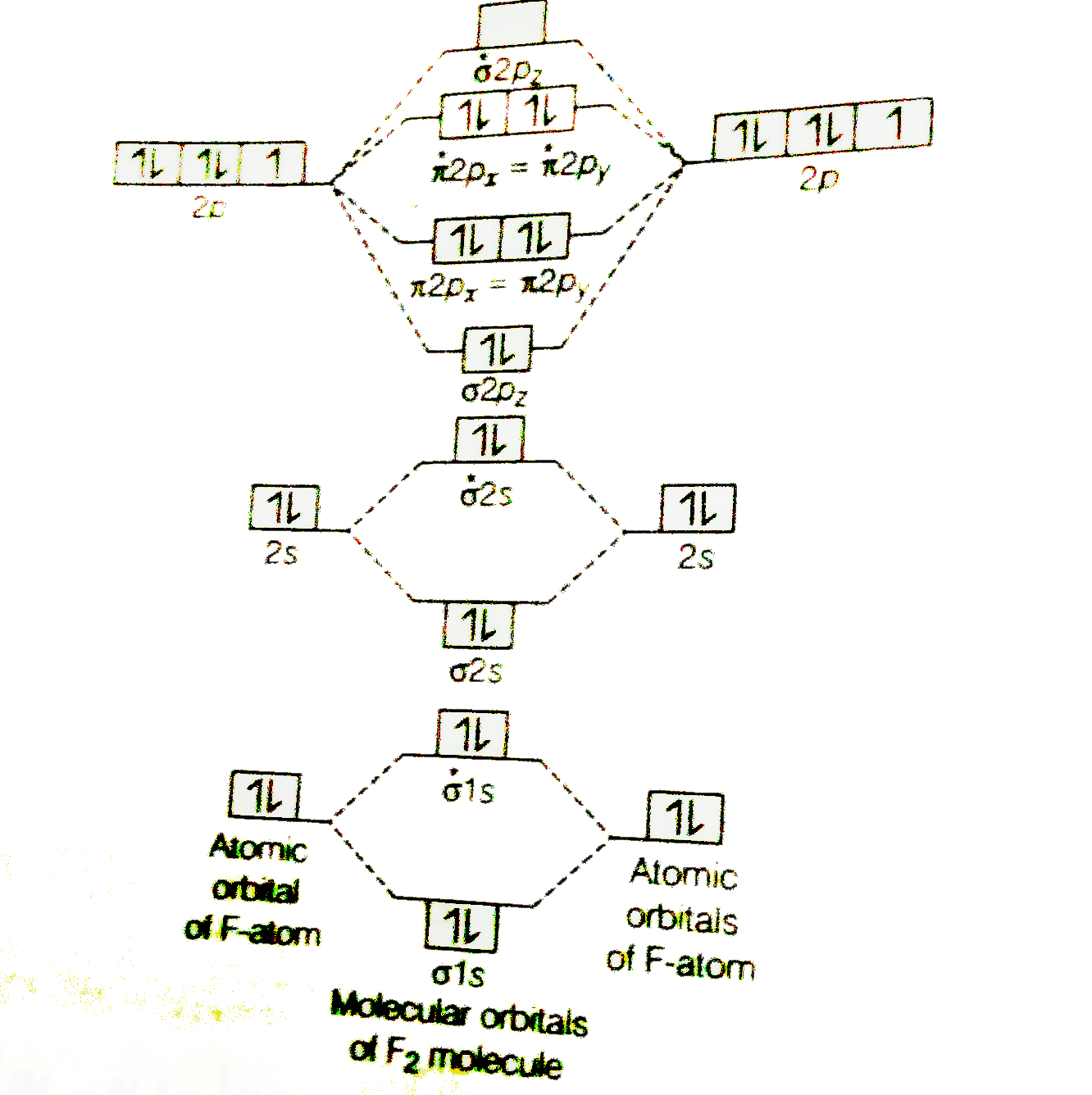
In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ... Molecular orbital diagram of b2. The next two would fill the 1 sigma e antibonding orbital. I can draw be2 but not this. B 2 molecule is formed by the overlap of atomic orbitals of both boron atoms. The electronic configuration of b atom z 5 is. It is diamagnetic due to the absence of any unpaired electron. Academia.edu is a platform for academics to share research papers. As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond. This example was covered in class to show the rare exception that this single bond is a bond.
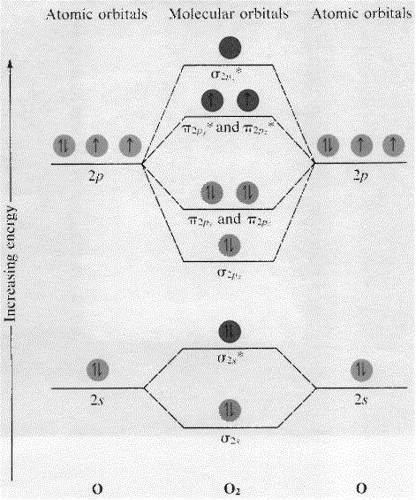
B2 molecular orbital diagram. Since bond order is zero be 2 molecule does not exist. This was on a quiz and i somehow got the bond order and the lumo indicated wrong. I also calculated the bond order of this molecule to be 32. B 2 molecule is formed by the overlap of atomic orbitals of both boron atoms. Department of Molecular & Cell Biology, Science Faculty: Thomas Scriba: TB vaccines and immunology: SATVI & Immunology, Department of Pathology: Edward Sturrock: Protein biochemistry, angiotensin-converting enzyme: Chemical & Systems Biology, Department of Integrative Biomedical Sciences: Digby Warner : Mycobacterial physiology & pathogenesis: Medical Microbiology, Department of Pathology ... 'Schistosomiasis induces plasma cell death in the bone marrow and suppresses the efficacy of anti-viral vaccination' & 'The immunological role of cell wall components from diverse Mycobacterium tuberculosis clinical isolates' - July 2021 In B2, C2, and N2, the 𝜎2𝑝 orbital is higher in energy than the 𝜋2𝑝 orbitals as shown in diagram A. In O2 and F2, the 𝜋2𝑝 orbitals are higher in energy than the 𝜎2𝑝 orbital as shown in diagram B. The number of valence electrons per atom of an element is related to the group number in the periodic table. Boron, in group 3A, has 3 valence electrons per atom. Diatomic ...

A Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic B 2 2 B2 C 2 2 B 2 2 And N 2 2 B Draw The Lewis Structures And Molecular Orbital Diagrams For
Pembuatan Senyawa kompleks asetial asetanoat. Enter the email address you signed up with and we'll email you a reset link.
Effusion of a 1:1 mixture of two gases, represented by unshaded and shaded spheres in the diagram below, through a small pinhole produces the result shown below. The shaded spheres have a molecular mass of 32 amu. Which gas molecules have the higher average speed and what is the molecular mass of the unshaded molecules?
14:24This video shows the end of the Be2 molecule MO diagram and explains pi orbitals, paramagnetism, and the ...26 Mar 2014 · Uploaded by Diego Troya

Draw The Molecular Orbital Diagram For I Be2 Ii B2 And Predict Bond Order And Magnetic Properties From Chemistry Chemical Bonding And Molecular Structure Class 11 Haryana Board English Medium
By drawing molecular orbital diagrams for b2 c2 n2 o2 and f2 predict which of these homonuclear diatomic molecules are magnetic. A number of valence electrons of each boron atom 3in the formation of b2 molecule three valence electrons of each boron atom ie. I drew a diagram of b2 in which i filled both bonding and anti bonding orbitals of 2s ...
According to molecular orbital theory, the atomic orbitals having comparable energy overlap and result in the formation of the same number of molecular orbitals. The molecular orbitals having the same sign combine and give bonding molecular orbitals. We have to draw the molecular orbital diagram for ${{\text{B}}_{\text{2}}}$ molecule.
9 Molecular Geometry and Bonding Theories 330 9.1 MOLECULAR SHAPES 332 9.2 THE VSEPR MODEL 334 Effect of Nonbonding Electrons and Multiple Bonds on Bond Angles 338 Molecules with Expanded Valence Shells 339 Shapes of Larger Molecules 342. 9.3 MOLECULAR SHAPE AND MOLECULAR POLARITY 343. 9.4 COVALENT BONDING AND ORBITAL OVERLAP 345
B2 molecule is formed by the overlap of atomic orbitals of both boron atoms. A number of valence electrons of each boron atom = 3. In the formation of B2 ... Rating: 4,4 · 740 votes · Free · Android · Educational
B2 molecular orbital diagram. This also causes a large jump in energy in the 2p σ orbital. For example when two hydrogen atoms bond a σ1s bonding molecular orbital is formed as well as a σ1s antibonding molecular orbital. Valence bond model vs. The molecular orbital diagram for an o 2 molecule would therefore ignore the 1s electrons on both ...
6:08This video discusses how to draw the molecular orbital (MO) diagram for the B2(-) molecule. The bond order ...2 Jun 2021 · Uploaded by Principia
23.10.2021 · Summary of licorice components properties: molecular weight (MW), number of H-bond donor/acceptor (HA/HD), water solubility (WS), and quantum chemical parameters, including the energy levels of the highest occupied molecular orbital (E HOMO), lowest unoccupied molecular orbital (E LUMO), energy gap (ΔE), dipole moment (μ), electronegativity (χ), global hardness (η) and the …
10:50From the periodic table as we have already discussed the Molecular orbital diagrams of diatomic molecules of ...24 Jun 2020 · Uploaded by Edmerls

Part A By Drawing Molecular Orbital Diagrams For B2 C2 N2 O2 And F2 Predict Which Of These Brainly Com
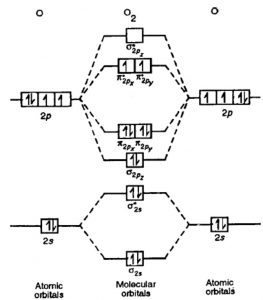
Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11

A Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic B 2 2 B2 C 2 2 B 2 2 And N 2 2 B Draw The Lewis Structures And Molecular Orbital Diagrams For












0 Response to "34 b2 molecular orbital diagram"
Post a Comment