34 solidus line phase diagram
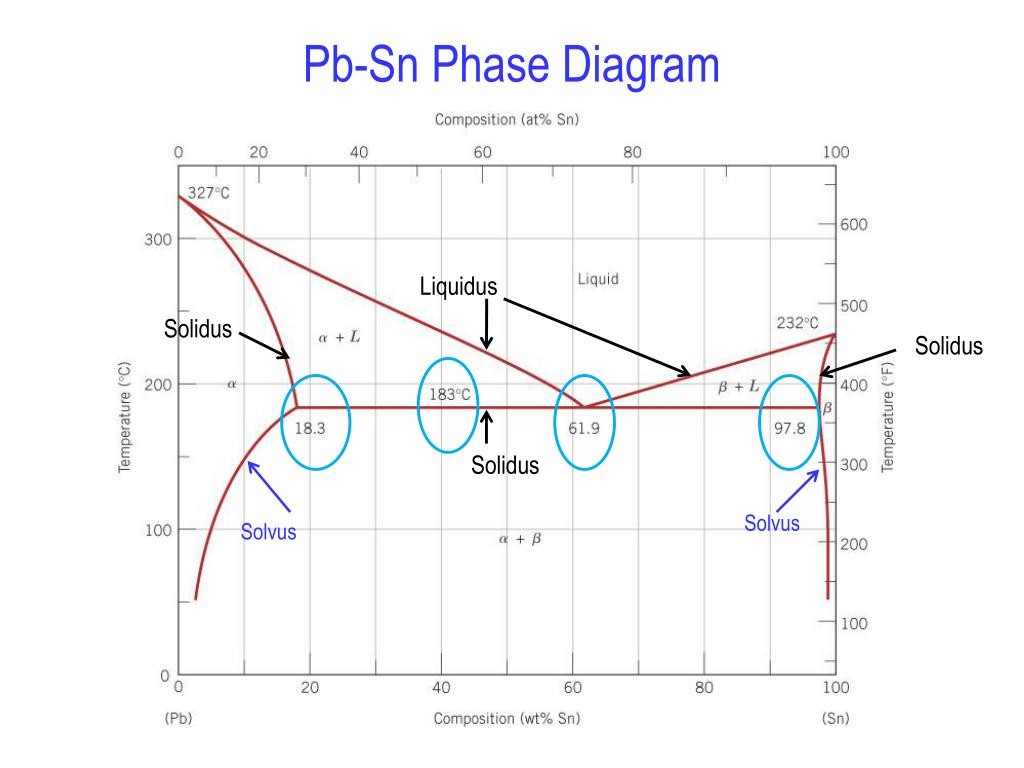
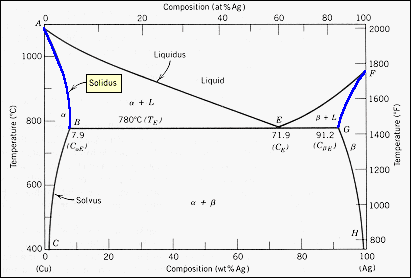
MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 3 Solvent - host or major component in solution,solute- minor component. Solubility Limitof a component in a phase is the maximum amount of the component that can be dissolved in 2-phase region: 1. Draw the tie line. 2. Note where the tie line intersects the liquidus and solidus lines (i.e. where the tie line crosses the phase boundaries). 3. Read off the composition at the boundaries: Liquid is composed of CL amount of Ni (31.5 wt% Ni). Solid is composed of Cαααα amount of Ni (42.5 wt% Ni).
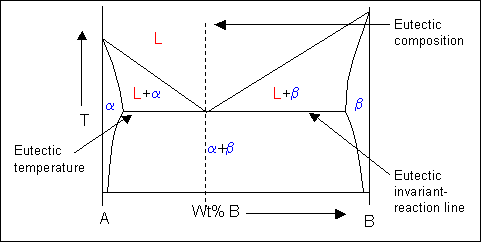
Liquidus - The line separating the field of all liquid from that of liquid plus crystals. Solidus - The line separating the field of all solid from that of liquid plus crystals. Eutectic point - the point on a phase diagram where the maximum number of allowable phases are in equilibrium. When this point is reached, the temperature must remain ...
Solidus line phase diagram
2. It is slowly heated until it reaches the solidus line. At the solidus line the system shifts laterally to the 30/70 eutectic point. 3. At the eutectic both components P and Q begin melting at the same time in the ratio of 30% P and 70% Q. Melting at the eutectic is always at the ratio of the eutectic, regardless of the starting composition. The solvus is represented by a line on a phase diagram that separates a solid phase from a solid1 + solid2 phase, where solid1 and solid2 are different microstructures. The eutectic is represented by the horizontal line in a eutectic binary phase diagram, connecting the intersections of the solidus and solvus lines from both sides. The eutectic ... mass fraction of each phase is 0.5, estimate: (a) The temperature of the alloy (b) The compositions of the two phases Solution (a) We are given that the mass fractions of α and liquid phases are both 0.5 for a 30 wt% Sn-70 wt% Pb alloy and asked to estimate the temperature of the alloy. Using the appropriate phase diagram, Figure 9.8, by trial
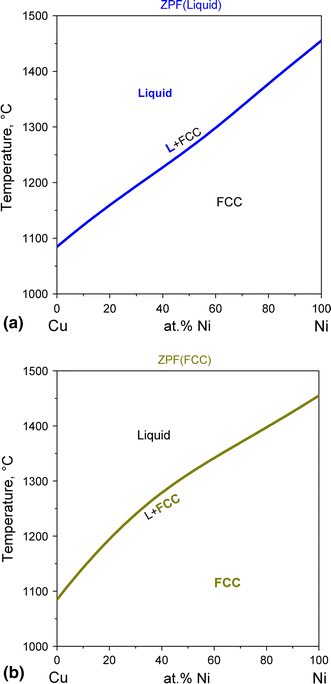
Solidus line phase diagram. The Ti-Si-N-O quaternary phase diagram • Entire phase diagram can be calculated by taking into account all possible combinations of reactions and products • 4 ternary diagrams of Ti-Si-N, Ti-N-O, Ti-Si-O and Si-N-O were evaluated • additional quaternary tie lines from TiN to SiO 2 and Si 2N 2O A.S.Bhansali, et al., J.Appl.Phys. 68(3 ... l i q u i d u s s o l i d u s A(1100,60) B (1 2 5 0, 3 5) Cu-Ni phase diagram A(1100, 60): 1 phase: B(1250, 35): 2 phases: L + Determination of phase(s) present Melting points: Cu = 1085°C, Ni = 1453 °C Solidus - Temperature where alloy is completely solid. Above this line, liquefaction begins. Liquidus - Temperature where alloy is completely ... 1. Locate composition and temperature in diagram . 2. In two phase region draw the tie line or isotherm . 3. Fraction of a phase is determined by taking the length of the tie line to the phase boundary for the other phase, and dividing by the total length of tie line . The lever rule is a mechanical analogy to the mass balance calculation. The ... Phase Diagram: In this phase diagram, which is typical of most substances, the solid lines represent the phase boundaries.The green line marks the freezing point (or transition from liquid to solid), the blue line marks the boiling point (or transition from liquid to gas), and the red line shows the conditions under which a solid can be converted directly to a gas (and vice-versa).
mass fraction of each phase is 0.5, estimate: (a) The temperature of the alloy (b) The compositions of the two phases Solution (a) We are given that the mass fractions of α and liquid phases are both 0.5 for a 30 wt% Sn-70 wt% Pb alloy and asked to estimate the temperature of the alloy. Using the appropriate phase diagram, Figure 9.8, by trial The solvus is represented by a line on a phase diagram that separates a solid phase from a solid1 + solid2 phase, where solid1 and solid2 are different microstructures. The eutectic is represented by the horizontal line in a eutectic binary phase diagram, connecting the intersections of the solidus and solvus lines from both sides. The eutectic ... 2. It is slowly heated until it reaches the solidus line. At the solidus line the system shifts laterally to the 30/70 eutectic point. 3. At the eutectic both components P and Q begin melting at the same time in the ratio of 30% P and 70% Q. Melting at the eutectic is always at the ratio of the eutectic, regardless of the starting composition.











0 Response to "34 solidus line phase diagram"
Post a Comment