35 d orbital energy diagram
• The energy increase of the e g orbitals and the energy decrease of the t 2g orbitals must be balancedrelative to the energy of the hypotheticalsphericalfield(aka the barycenter).• The energy of each of the two orbitals of the e g set rises by +3/5 o (+6 Dq) while the energy of eachof the three t 2g orbitalsfallsby ‐2/5 o(‐4Dq). • Thisresults inno netenergy changefor the system: Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
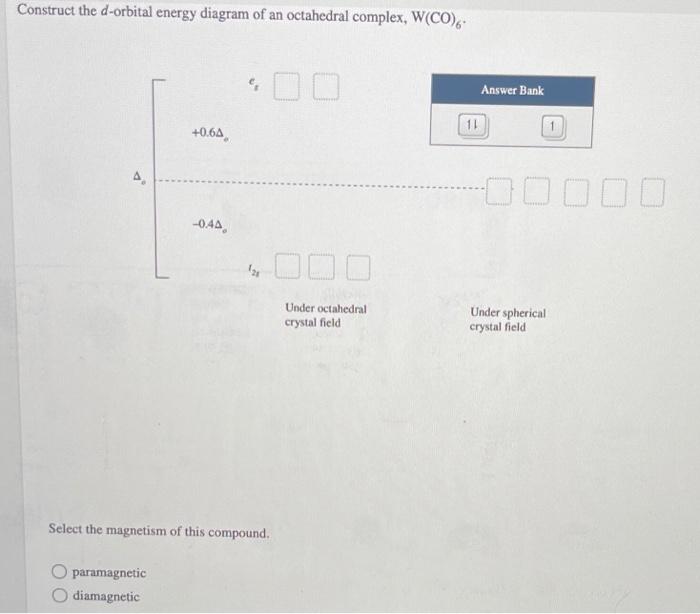
Crystal Field Splitting in an Octahedral Field eg Energy 3/5 o o 2/5 o t2g e g - The higher energy set of orbitals (d z2 and d x2-y2) t 2g - The lower energy set of orbitals (d xy, d yz and d xz) Δ o or 10 Dq - The energy separation between the two levels The eThe eg orbitals are repelled by an amount of 0 6orbitals are repelled by an amount of 0.6 Δo The t2gorbitals to be stabilized to the ...

D orbital energy diagram
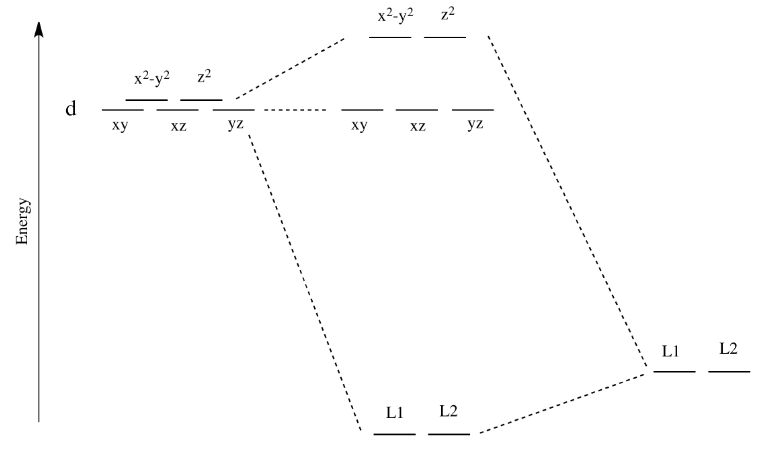
A molecular orbital is a region of space in a covalent species where electrons are likely to be found. The combination of two atomic orbitals always forms two molecular orbitals; the bonding molecular orbital, which is _____ in energy, and the antibonding molecular orbital, which is _____ in energy, than the original atomic orbitals. For example, the orbital 1s (pronounced as the individual numbers and letters: "'one' 'ess'") is the lowest energy level (n = 1) and has an angular quantum number of ℓ = 0, denoted as s. Orbitals with ℓ = 1, 2 and 3 are denoted as p, d and f respectively. D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns: 1. Octahedral 2. Tetrahedral 3. Trigonal bipyramidal 4. Square pyramidal d z2x2-y d xy d yzxz 5. Square planar d z2x2-y d xy d yzxz d z2 d x2-yxy d yz d xz d z2 d x2-y2 d xy d yz d xz d z2 d x2-y d xy d yz d xz z x y
D orbital energy diagram. Each orbital is denoted by three quantum numbers, n, l and m. The number against an orbital is indicated by the energy level of the electron in that orbital. The energy level closest to the nucleus is 1; the next one is 2 and so on. The symbols of the orbital shapes i.e. s, p, d and f come from the words meaning sharp, diffuse, principal and ... Figure 46. The splitting pattern of d-orbital energy levels of d3-metal complexes in tetragonal distortion. 2. Rhombic distortion: The unequal amount of elongation or compression along two four-fold axes of rotation in octahedral complexes produces rhombic distortions. The common examples of rhombic distortion are high- Answer (1 of 4): Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first. 2. Pauli Exclusion Principle: Only 2 electrons per orbital, they must have opposite spin. 3. Hund's Rule: Given sev... The overall molecular orbital energy level diagram for σ-bonding in octahedral complexes can be shown as: Figure 10. The formation of σ-molecular orbitals (bonding, antibonding and non-bonding) in octahedral complexes of transition metals. Buy the complete book with TOC navigation,
Draw the d orbital diagrams for the high spin and the low spin case for each ion. Problem CC8.5. Usually, tetrahedral ions are high spin rather than low spin. Explain why. Problem CC8.6. The d orbital splitting diagram for a square planar environment is shown below. This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... d-orbital diagram for [Fe(H 2 O) 6] 3+: The first three electrons go into t 2g orbitals unpaired. The 4th and 5th electrons must choose whether to pair up with electrons already in t 2g (which costs energy) or to go into higher energy e g orbitals (which also costs energy). In this case, the splitting energy is less than the pairing energy so the 4th and 5th electrons go into the e g orbitals. Similar to s orbitals, size, and energy of p orbitals increases with an increase in the principal quantum number (4p > 3p > 2p). The Shape of d Orbitals. The magnetic orbital quantum number for d orbitals is given as (-2,-1,0, 1,2). Hence, we can say that there are five d-orbitals. These orbitals are designated as d xy, d yz, d xz, d x 2 –y 2 ...
Energy level diagram for Molecular orbitals. ... If N b = Na,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding molecular orbitals. 2) Stability of molecules in terms of bond order. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... Square planar d z2x2-y d xy d yzxz d z2 d x2-yxy d yz d xz d z2 d x2-y2 d xy d yz d. A general d-orbital splitting diagram for square planar (D 4h) transition metal complexes can be derived from the general octahedral (O h) splitting diagram, in which the d z 2 and the d x 2 −y 2 orbitals are degenerate and higher in energy than the degenerate set of d xy, d xz and d yz orbitals. Orbital diagram of Nitrogen (N) 8. Orbital diagram of Oxygen (O) 9. Orbital diagram of Fluorine (F) 10. Orbital diagram of Neon (Ne) 11. Orbital diagram of Sodium (Na)
Chemistry questions and answers. Using crystal field theory, draw an electron box energy level diagram for the valence d orbital on the cobalt atom in a [CoBr_6]^3- complex. Your diagram should show the relative energy of each orbital, and the number of electron in each orbital.
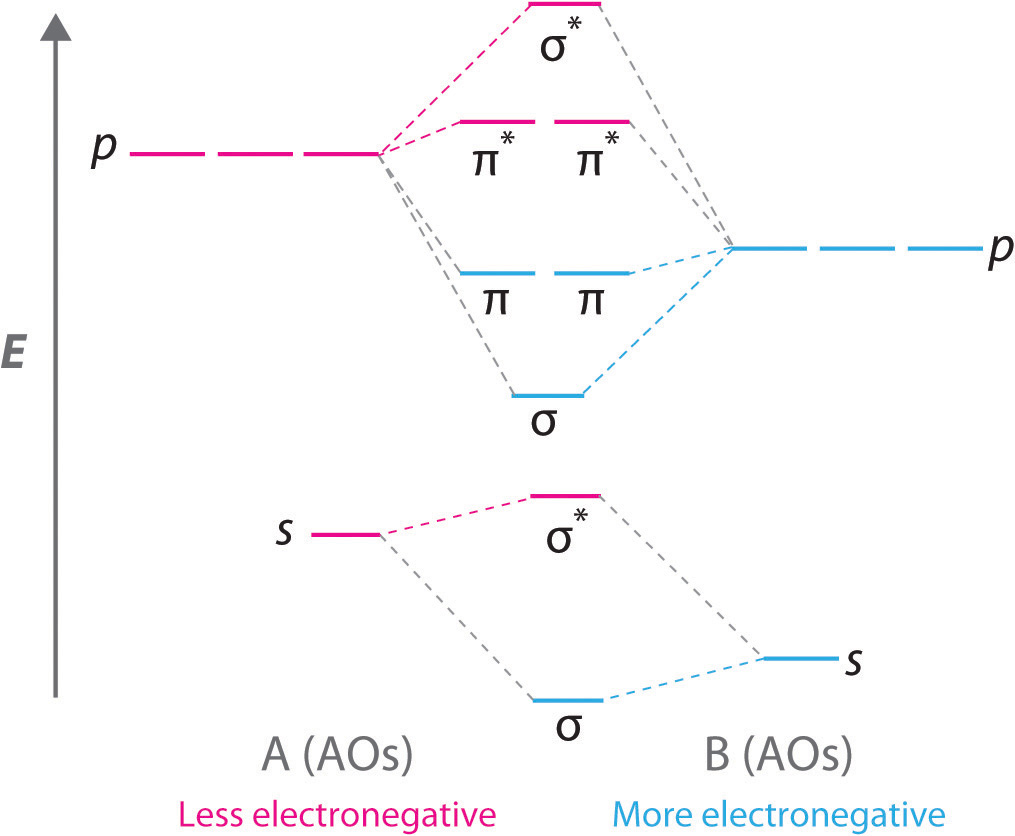
In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ...
A 3s orbital is even larger, and it has three nodes. p ORBITALS. Not all electrons inhabit s orbitals. At the first energy level, the only orbital available to electrons is the 1s orbital. However, at the second level, there are also orbitals called 2p orbitals in addition to the 2s orbital.
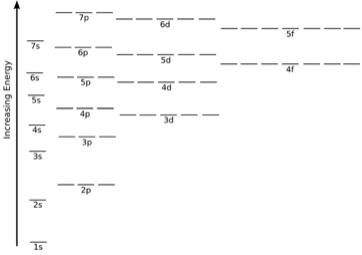
For example, when I show you the electron configuration for oxygen (1s²2s²2p⁴), this means that the 1s orbital is lowest in energy, followed by the 2s orbital and 2p orbital, respectively. 1, 3, 5, 7. This is shorthand for the rule that energy levels hold one s-orbital, three p-orbitals, five d-orbitals, and seven s-orbitals.

Draw Figure To Show Splitting Of D Orbitals In An Octahedral Crystal Field From Chemistry Coordination Compounds Class 12 Cbse
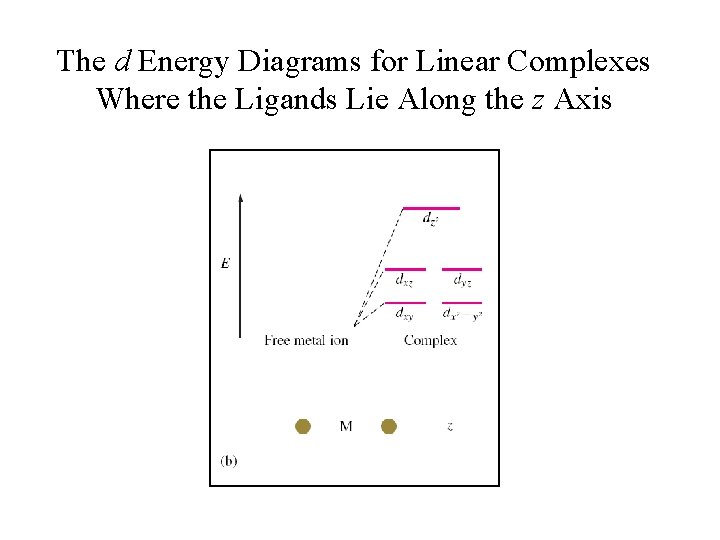
Sketch a d-orbital energy diagram for the following. a. a linear complex ion with ligands on the x axis. b. a linear complex ion with ligands on the y axis. Step-by-step solution. Step 1 of 5. A complex is composed with a central metal atom, and ligands. Linear complex is one in which central atom and ligands present in a straight line.

Drawing A Crystal Field Theory Energy Level Diagram Using Crystal Field Theory Draw An Electron Box Homeworklib
There are five 3d orbitals called. 3dxy. 3dxz. 3dyz. 3dx2- y2. 3dz2. To make sense of it, we need to look at these in two groups: 3dxy, 3dxzand 3dyz. The names tell you that these orbitals lie in the x-y plane, the x-z plane and the y-z plane respectively.
Square Planar D Orbital Splitting Diagram. Crystal Field Theory (CFT) is a model that describes the breaking of degeneracies of electron In a tetrahedral crystal field splitting, the d-orbitals again split into two groups, with an energy difference of Δtet. The lower energy Square planar and other complex geometries can also be described by CFT.
Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms.
with the. d. orbital being one level lower than the energy level it is on. orbital diagram for arsenic see more ideas approximately from diagram electron configuration gallery & create your house design images related to pictures as well help you in locating the solution are seeking about itOrbital diagram for arsenic.
Electron configuration of sodium atom through orbital diagram. Atomic energy levels are subdivided into sub-energy levels. These sub-energy levels are called orbital. The sub energy levels are expressed by 'l'. The value of 'l' is from 0 to (n - 1). The sub-energy levels are known as s, p, d, f.
This video explains s, p, d, and f orbitals, sublevels, and their shapes. It discusses the 4 quantum numbers n, l, ml, and ms. n represents the energy leve...
Jul 08, 2021 · According to this, the d z 2 orbital should be raised in energy by ( 1 / 4) + ( 1 / 4) + ( 1 / 4) + ( 1 / 4) = 1 e σ, and d x 2 − y 2 should be raised by ( 3 / 4) + ( 3 / 4) + ( 3 / 4) + ( 3 / 4) = 3 e σ. The rest of the orbitals should stay the same energy they are as their coefficients are zero. Is this a correct diagram for a planar square coordination complex, with sigma only ligands, such as [ P t ( N H X 3) X 4] X 2 X +?
Fig. d-orbital splitting in an octahedral crystal field. The formation of complex depend on the crystal field splitting, ∆ o and pairing energy (P). i)If ∆ o < P, the fourth electron enters one of the eg orbitals giving theconfiguration t 2g 3. Ligands for which ∆ o < P are known as weak field ligands and form high spin complexes.
Chemistry questions and answers. Construct the d-orbital energy diagram of octahedral complex [CoCl_6]^4-. How many unpaired electrons are there in [CoCl_6]^4-? Calculate the Ligand Field Stabilization Energy (LFSE) of this octahedral complex with respect to delta_0. Question: Construct the d-orbital energy diagram of octahedral complex [CoCl_6 ...
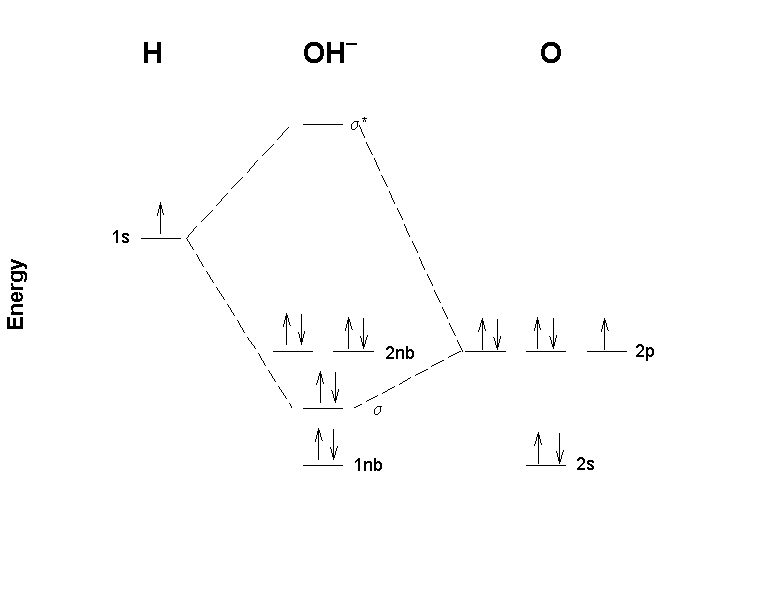
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
For example, l = 0 is the s orbital, l = 1 is the p orbital, l = 2 is the d orbital, l = 3 is the f orbital, and so on. The general rule is the energy increases with the principal quantum number and azimuthal quantum number; however, there are some exceptions.
A qualitative molecular orbital diagram for ferrocene (D 5d) FeII Fe SALC's p p a 2u * e 1u * x e 1u z y a e 2g * e 2g, u a 2u, e 1u a 1g * u a 1g e 1g * LUMO a 1g, e 1g, e 2g e 2g a 1g HOMO dyz dxz e 1g e 1g e 1u e 1g, e 1u a 1g a 2u a 1g, a 2u • Due to a difference in energies the lowest energy a 1g molecular orbital is mainly ligand based ...
D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns: 1. Octahedral 2. Tetrahedral 3. Trigonal bipyramidal 4. Square pyramidal d z2x2-y d xy d yzxz 5. Square planar d z2x2-y d xy d yzxz d z2 d x2-yxy d yz d xz d z2 d x2-y2 d xy d yz d xz d z2 d x2-y d xy d yz d xz z x y
For example, the orbital 1s (pronounced as the individual numbers and letters: "'one' 'ess'") is the lowest energy level (n = 1) and has an angular quantum number of ℓ = 0, denoted as s. Orbitals with ℓ = 1, 2 and 3 are denoted as p, d and f respectively.
A molecular orbital is a region of space in a covalent species where electrons are likely to be found. The combination of two atomic orbitals always forms two molecular orbitals; the bonding molecular orbital, which is _____ in energy, and the antibonding molecular orbital, which is _____ in energy, than the original atomic orbitals.

Scielo Brasil Explaining The Geometry Of Simple Molecules Using Molecular Orbital Energy Level Diagrams Built By Using Symmetry Principles Explaining The Geometry Of Simple Molecules Using Molecular Orbital Energy Level Diagrams Built

Draw An Orbital Energy Level Diagram Showing The Configuration Of The D Electrons On The Metal Ion In The Complex Fe Cn 63 Study Com



















0 Response to "35 d orbital energy diagram"
Post a Comment