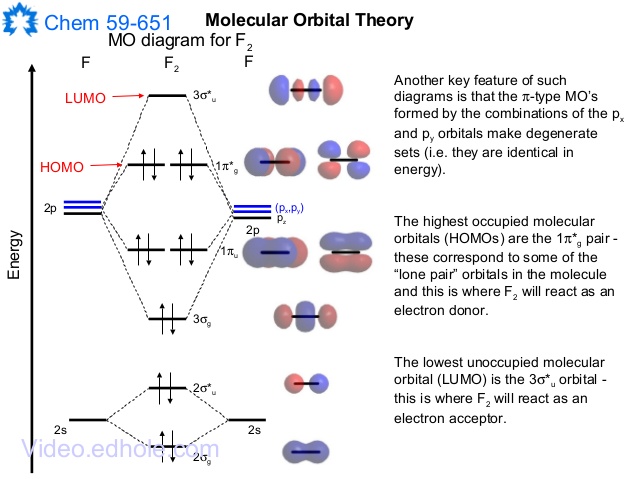
35 h2- molecular orbital diagram
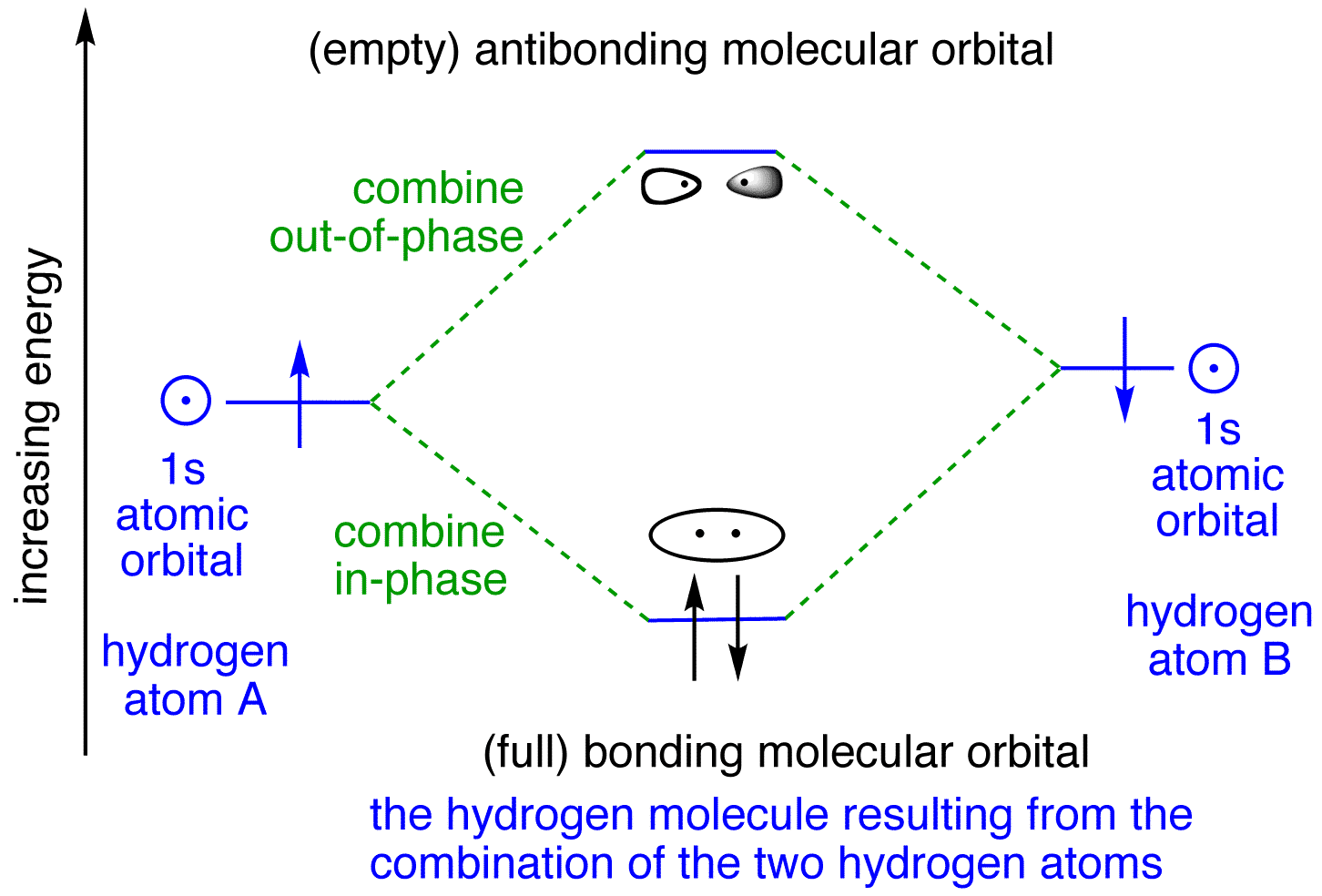
Sketch the molecular orbitals of the h2 ion and draw its energy level diagram. Molecular orbitals of h 2 the hydrogen atom is the simplest atom and its molecule ceh2 is also the simplest molecule than monoatomic molecules such as cehe and cene etc. Problem 1 since both molecular ions have a bond order of 12 they are approximately equally stable. Hydrogen Molecular Orbital Diagram Atomic hydrogen has 1 electron in a 1s orbital. Of course, there are 2s, 2p, 3s, 3p, etc. empty orbitals at higher energy. Let's just consider the 1s orbitals. Remember that an orbital is a mathematical function that describes the probability of finding an electron in space.
4:39This video discusses how to draw the molecular orbital (MO) diagram for the H2(2-) molecule. The bond ...Jan 14, 2021 · Uploaded by Principia

H2- molecular orbital diagram
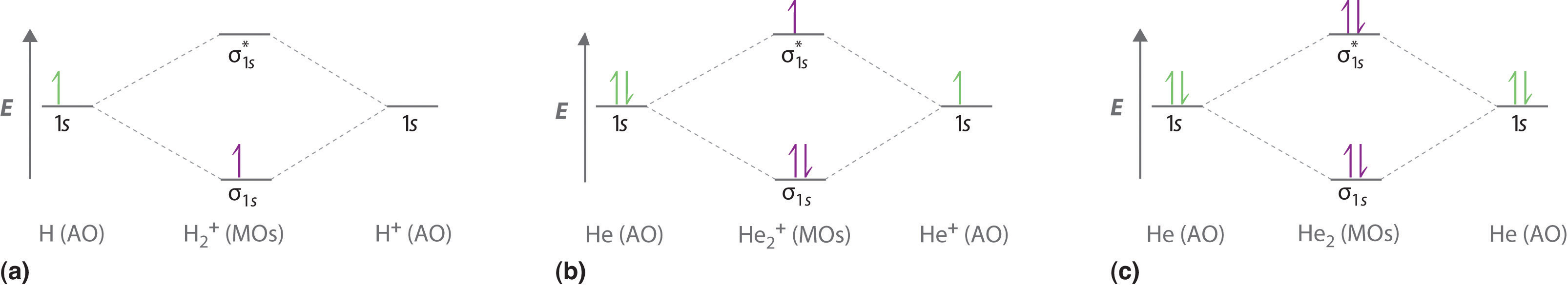
1. Problem: Draw MO energy diagrams for the molecular ions H2+ and H Since both molecular ions have a bond order of 1/2, they are approximately equally.Solution: Construct the molecular orbital diagram for He2 + and then identify the bond order. Problem Construct the molecular orbital diagram for He 2 + and then identify the bond order. In this video, we take a detailed look at the molecular orbitals of the H2 molecule, with an introduction to molecular orbital diagrams. Discussed in this v... Mo · Construct The Molecular Orbital Diagram For H2 And Then Identify The Bond Order. chemical bonding molecular orbitals of h2 and he2 as before the greater the number of these nodal planes the more the electrons that occupy the orbitals are excluded from the region between the nuclei and hence the higher the energy the resulting molecular ...
H2- molecular orbital diagram. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic ... Spin‐orbitals of type 1 and 3 have the same symmetry, and therefore can "mix" (to give improved wavefunctions and energy eigenvalues): 1 ψψ αβ ... Answer (1 of 2): In order to predict the bond order, molecular orbital diagram for H2- is to be drawn. According to MOT number of atomic orbitals combined is equal to total number of molecular orbitals formed.Electronic configuration of H is 1s1. when two hydrogen atoms come closer, then on combi... 7:03Short lecture on the molecular orbitals of the hydrogen molecule.The 1s orbitals of each hydrogen atom ...Aug 15, 2016 · Uploaded by TMP Chem
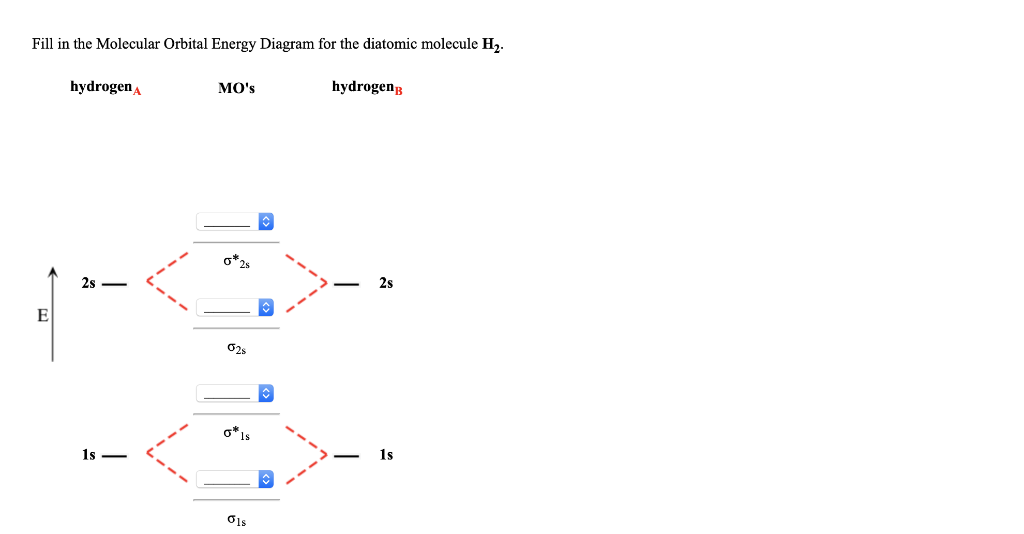
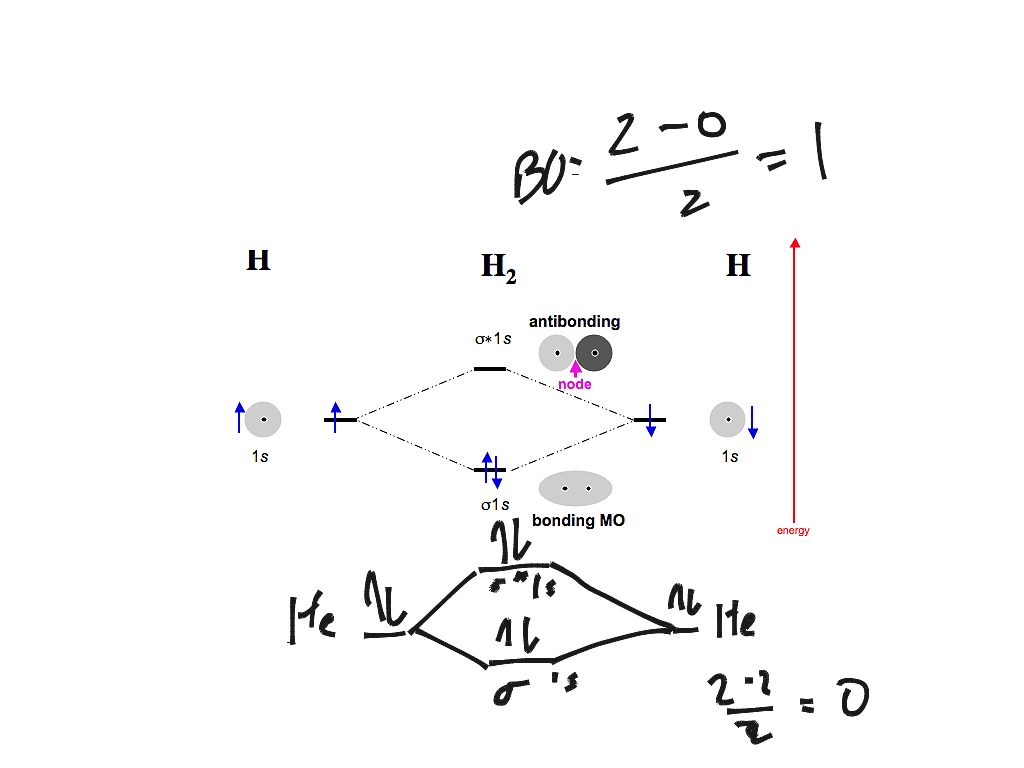
Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here. Chemical bonding - Molecular orbitals of H2 and He2: The procedure can ... 3:47This video discusses how to draw the molecular orbital (MO) diagram for the diatomic hydrogen or H2 ...Jan 1, 2021 · Uploaded by Principia Feb 3, 2021 — For H2, bond order = 1/2 (2-0) = 1, which means H2has only one bond. The antibonding orbital is empty. Thus, H2 is a stable molecule. Again, in ... H2 molecular orbital diagram. Qualitative mo theory orbital diagram for homonuclear diatomics. The bond order of a diatomic molecule is defined as one half the difference between the number of electrons in bonding orbitals nb and the number of electrons in antibonding orbitals na. A double covalent bond a bond order of two.
Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [(2 − 2)/2 = 0], and we can make H+. of the orbitals is specified. b. Molecular Orbital Picture We are now in a position to discuss the basic principles of the molecular orbital (MO) method, which is the foundation of the electronic structure theory of real molecules. The first step in any MO approach requires one to define an effective one electron Hamiltonian, hˆ eff. To this ... 5:31He has two electrons with electronic configuration 1s2. Thus He molecule will have total 4 electrons and its ...Jun 8, 2020 · Uploaded by Edmerls Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ...
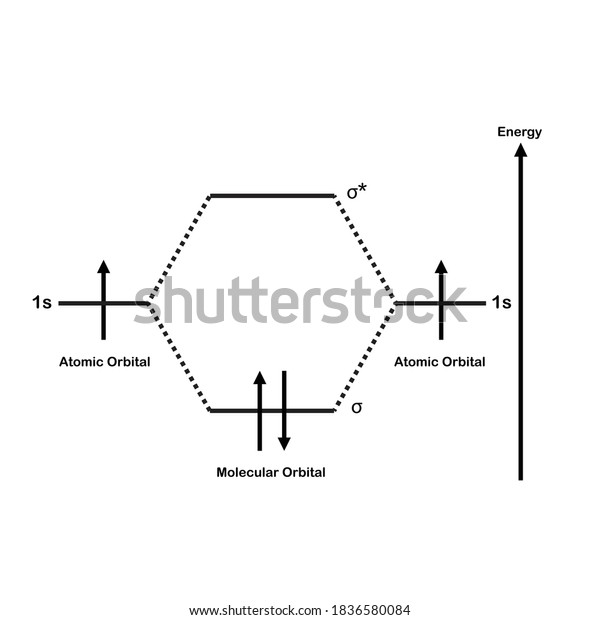
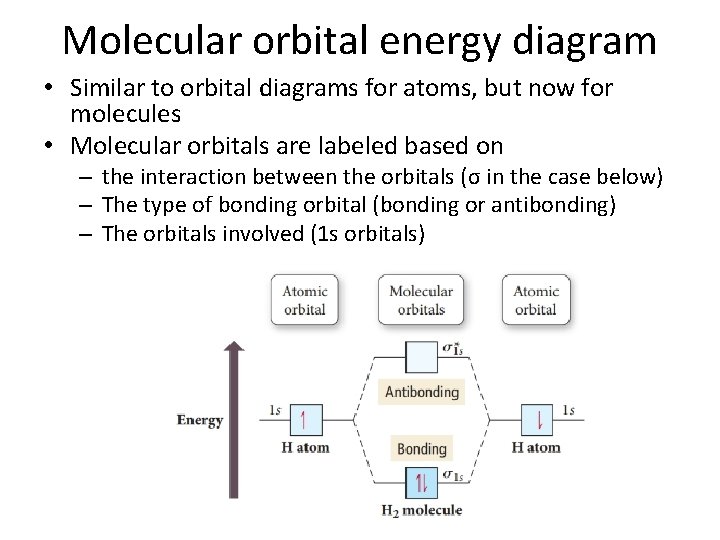
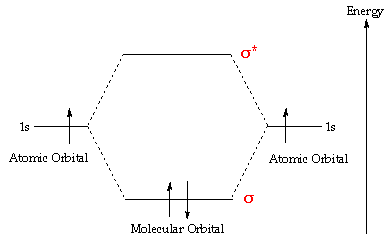
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.The Hydrogen Molecule Ion H2+Molecular Orbital Diagrams of Diatomic Molecules - Chem
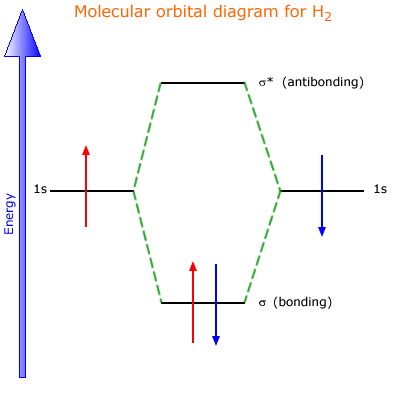
Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me ...
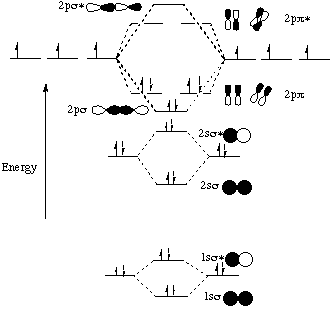
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
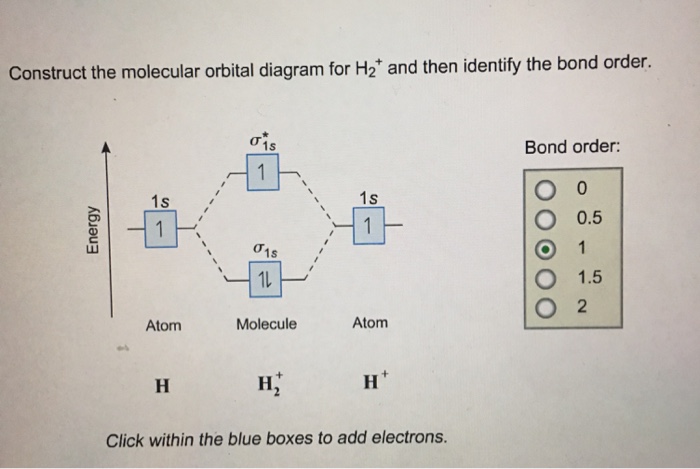
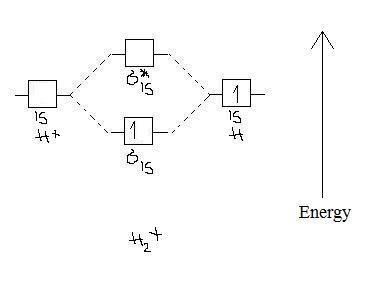
Chemistry questions and answers. Construct the molecular orbital diagram for H2+ If all of the orbitals are unoccupied, place the corresponding token in the bin underneath the answer bank. os Answer Bank 11 1 1s 1s Energy 015 all of the orbitals are unoccupied Atom Molecule Atom HT h H Identify the bond order. 0 0.5 1 1.5 2.
14+ H2 Molecular Orbital Diagram. A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals.
Molecular orbital diagram h2. Because of their simplicity they have been extensively studied. Two superpositions of these two orbitals can be formed one by summing the orbitals and the other by taking their difference. Construct the molecular orbital diagram for h2 and then identify the bond order.
Here is a complete overview of the bond order of h2. Bond order from Number of electrons in bonding and anti-bonding molecular orbits: Before knowing the Bond order of H 2 we should know about the molecular orbitals. When all the electrons occupying bonding molecular orbitals (N b) and anti-bonding molecular orbitals (N *) are counted separately the following cases arise:
chemical bonding - chemical bonding - Molecular orbitals of H2 and He2: The procedure can be introduced by considering the H2 molecule. Its molecular orbitals are constructed from the valence-shell orbitals of each hydrogen atom, which are the 1s orbitals of the atoms. Two superpositions of these two orbitals can be formed, one by summing the orbitals and the other by taking their difference.
Description of the molecular orbitals of the h2 molecule with an introduction to molecular orbital diagrams. Each boron atom has one 2s and three 2p valence orbitals. In order to predict the bond order molecular orbital diagram for h2 is to be drawn. Molecular orbitals of h 2 the molecular orbital approach is one explanation for the ceh h bond.
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
Science. Chemistry. Chemistry questions and answers. Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes to add electrons. Question: Construct the molecular orbital diagram for H2- and then identify the bond order.
4:32This video discusses how to draw the molecular orbital (MO) diagram for the H2- ion. The bond order of H2 ...Jan 3, 2021 · Uploaded by Principia
brings only a 1s orbital to the mixing. Just as in the molecules H2 and H2 +, the molecular orbitals for He2 are created by the combination of two 1s atomic orbitals (Fig. 1.46). Each helium atom brings two electrons to the molecule, though, so the electronic occupancy of the orbitals will be different from that for H2 or H2 +. The
This problem has been solved! A.Construct the molecular orbital diagram for H2^2+ and then identify the bond order. B.Construct the molecular orbital diagram for H2 and then identify the bond order. C.Construct the molecular orbital diagram for H2^- and then identify the bond order. D.Construct the molecular orbital diagram for H2^+ and then ...
Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals.
Molecular orbitals of h2 and he2. The molecular orbital energy level diagram for the h2 ion. Suppose that the ion is excited by light so that an electron moves from a lower energy to a higher energy molecular orbital. πε and jr k r mr lr defined explicitly in atkins. Sketch the molecular orbitals of the h2 ion and draw its energy level diagram.
Mo · Construct The Molecular Orbital Diagram For H2 And Then Identify The Bond Order. chemical bonding molecular orbitals of h2 and he2 as before the greater the number of these nodal planes the more the electrons that occupy the orbitals are excluded from the region between the nuclei and hence the higher the energy the resulting molecular ...
In this video, we take a detailed look at the molecular orbitals of the H2 molecule, with an introduction to molecular orbital diagrams. Discussed in this v...
1. Problem: Draw MO energy diagrams for the molecular ions H2+ and H Since both molecular ions have a bond order of 1/2, they are approximately equally.Solution: Construct the molecular orbital diagram for He2 + and then identify the bond order. Problem Construct the molecular orbital diagram for He 2 + and then identify the bond order.














0 Response to "35 h2- molecular orbital diagram"
Post a Comment