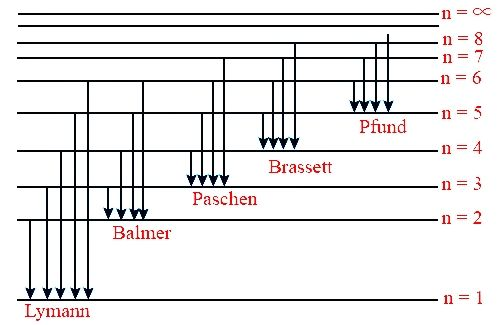
37 calculating the wavelength of a spectral line from an energy diagram
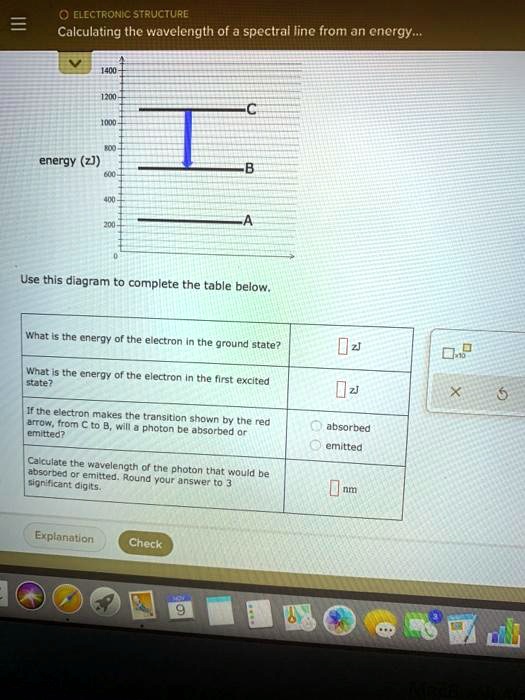
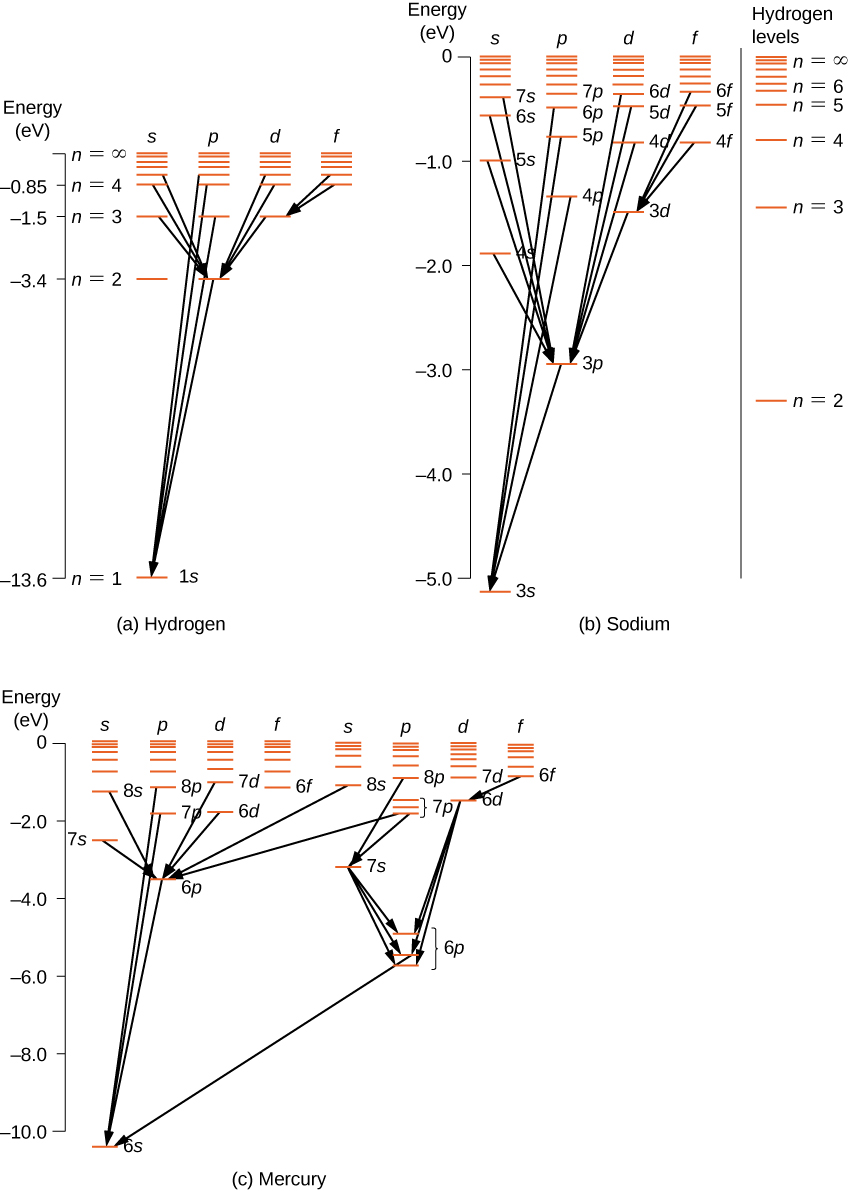
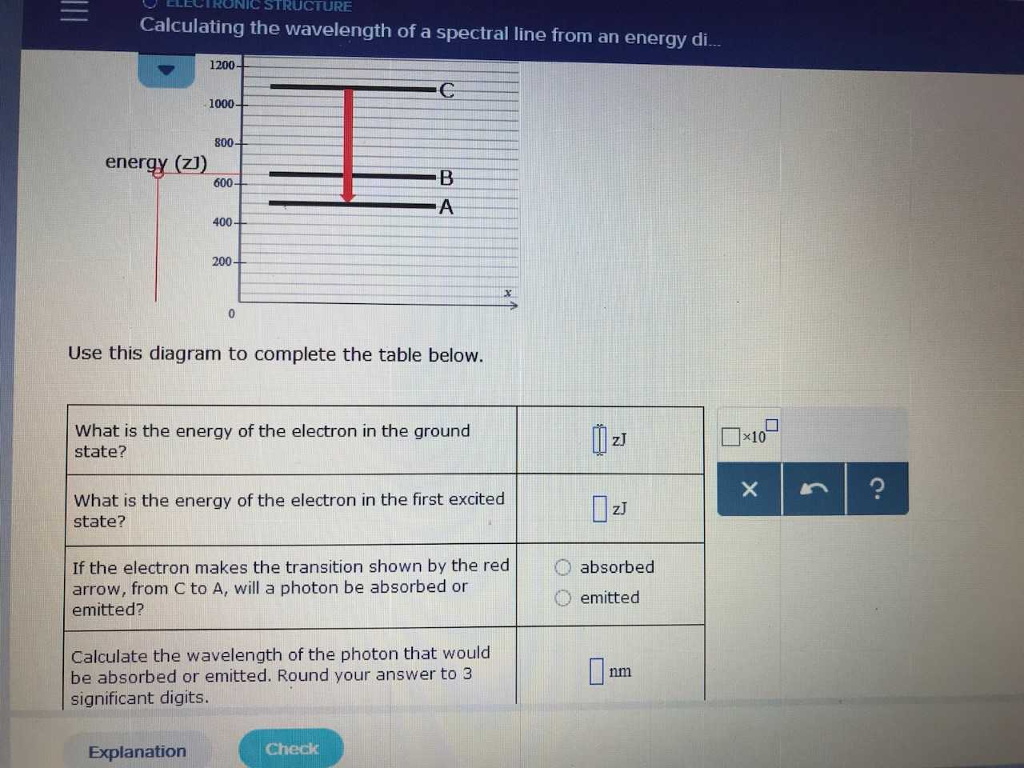
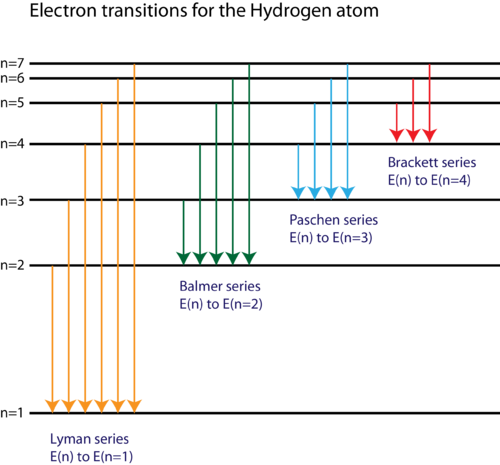
The basic hydrogen energy level structure is in agreement with the Bohr model. Common pictures are those of a shell structure with each main shell The energy levels agree with the earlier Bohr model, and agree with experiment within a small fraction of an electron volt. If you look at the hydrogen... ...Calculating the wavelength of a spectral line from an energy: ene Use this diagram to complete the table below What Is the energy of the 150 zJ Dap What is the energy of the electron In the first exclted state? 450 z] If the electron makes the transition shown by the red amtow from € to B will...
The wavelength calculator can assist you in determining the relationship between frequency and wavelength. Frequency (f) of a wave refers to how many times (per a given time duration) the particles of a medium vibrate when the wave passes through it.

Calculating the wavelength of a spectral line from an energy diagram
The reflected energy is referred to as the 'spectral content'. The differences in the spectral reflectance, which can be When the wavelength of incident energy is much smaller than the surface height variations or the particle sizes that make up a surface, the reflection from the surface is diffuse. Frequency and Wavelength Calculator, Light, Radio Waves, Electromagnetic Waves, Physics. where 'c' is the speed of light in meters per second, the Greek letter lambda λ is the wavelength in meters Also, the photon energy can be calculated by the formulas: where 'e' is energy (joules), 'f'... The authors say that the vertical line represents the average emission wavelength. If say hypothetically I had a function to represent this graph, how would I I believe that you want to find the wavelength such that the area of the function with wavelengths shorter than the average is equal to the area of...
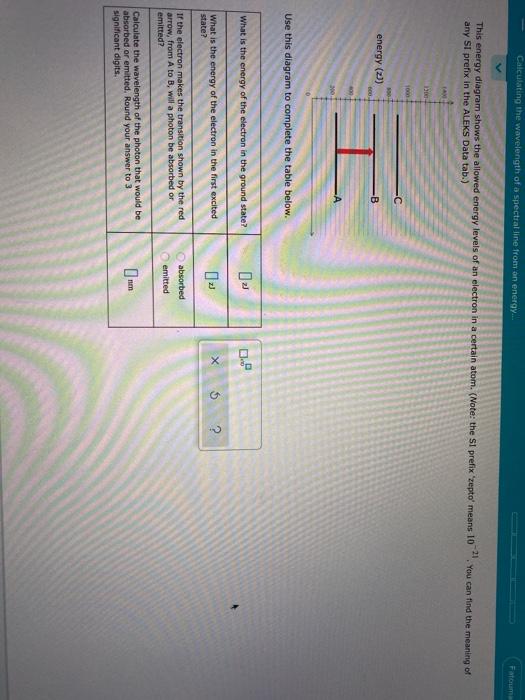
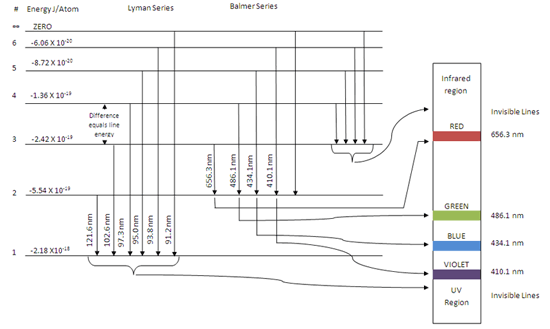
Calculating the wavelength of a spectral line from an energy diagram. 1/14/2017 ALEKS ; 1/4 Student Name: Joseph Lee Date: 01/14/2017 Electronic Structure Calculating the wavelength of a spectral line from an energy diagram This energy diagram shows the allowed energy levels of an electron in a certain atom. Therefore, you can calculate the energy of one mole of photons from either the light's wavelength or frequency. Identify the wavelength or frequency of the beam of light. You normally state wavelength in nanometers (nm) and convert it to meters for energy calculation purposes. no_spectral_lines = (Quantum Number*(Quantum Number-1))/2 Go. Energy of stationary state of hydrogen. How to calculate Wavelength of all spectral lines using this online calculator? Here is how the Wavelength of all spectral lines calculation can be explained with given input values... 8.4fCalculating the wavelength of a line in the spectrum of hydrogen.
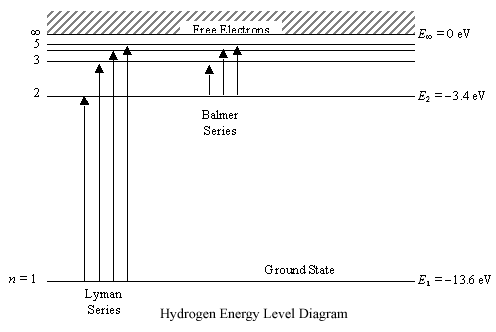
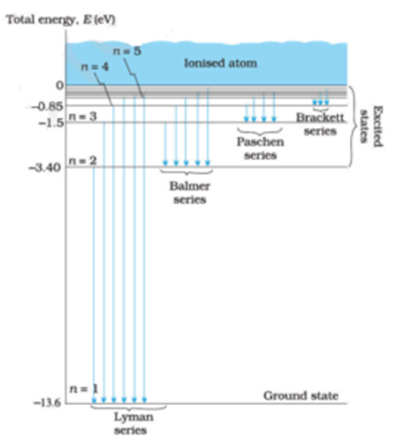
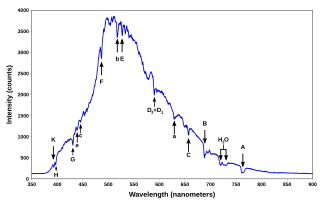
Spectral-line emission and absorption are intrinsically quantum phenomena. Unlike idealized waves, real radio waves do not have a continuum of possible energies. A second quantum effect important to spectral lines, particularly at radio wavelengths where hν≪kT. The spectral lines in atomic hydrogen and helium were then measured and their corresponding wavelengths calculated using the data This energy gives rise to a particular line in the spectrum. The formulation of various series in the Hydrogen spectrum is shown by an energy level diagram. 2 Calculating Wavelength Given Energy of a Photon. Wavelength is the distance of 1 frequency wave peak to the other and is most commonly associated with the electromagnetic spectrum.[1] X Research source Calculating wavelength is dependent upon the information you are given. An energy-level diagram for a hydrogen atom and several possible atomic transitions are shown in From a knowledge of the temperature and density of a gas, it is possible to calculate the fraction of the atom, the energy levels of the ion, and thus the wavelengths of the spectral lines it can produce...
We present spectral line wavelengths, identifications, and intensities in the 171-211 and 245-291 Å ranges from five solar plasma regions recorded by the Extreme-Ultraviolet Imaging Spectrometer (EIS) on Hinode. The recorded data were emitted from a quiet region, two active areas on the solar disk, a... Calculation Theory. Photon Energy and Wavelength. The power of short wavelength light sources in the EUV to X-ray range are often expressed as photon flux ( F ) in the units For any given flux and wavelength (in nm), equation 5 can be used to calculate the corresponding average power (in m W). Calculating wavelength of a wave. EM Spectrum (3 of 3) Calculate Energy and Frequency... Energy from Wavelength: Electromagnetic Calculate the wavelength, and nanometers, of the spectral lines produced when an electron in a hydrogen atom undergoes a transition from energy... The wavelength of a spectral line emitted by a hydrogen atom in the Lyman series is 16/15R cm. If an electron moves from one energy level to another, it radiates (or has to adsorb) a photon of energy equal to the difference of the two energy levels.
Calculating the wavelength of a spectral line from an energy diagram. First, the state with the lowest possible energy is called the ground Since the difference in energy between states A and B is =−600.zJ+400.zJ=200.zJ , the photon absorbed during the transition must have an energy of 200.zJ .
Solved Bad Man Man Aleks In Co O Electronic Structure Calculating The Wavelength Of A Spectral Line From An Energy This Energy Diagram Shows Course Hero
Emission-line spectra. Low-density clouds of gas floating in space will emit emission lines if they We can depict an atomic transition graphically by drawing a little ball on the diagram to represent the As mentioned earlier, the energy of a photon determines its wavelength. You can convert from one to...

Calculating The Wavelength Of A Spectral Line From An Energy Diagram Calculating The Wavelength Of A Spectral Line From An Energy Diagram The Lowest Course Hero
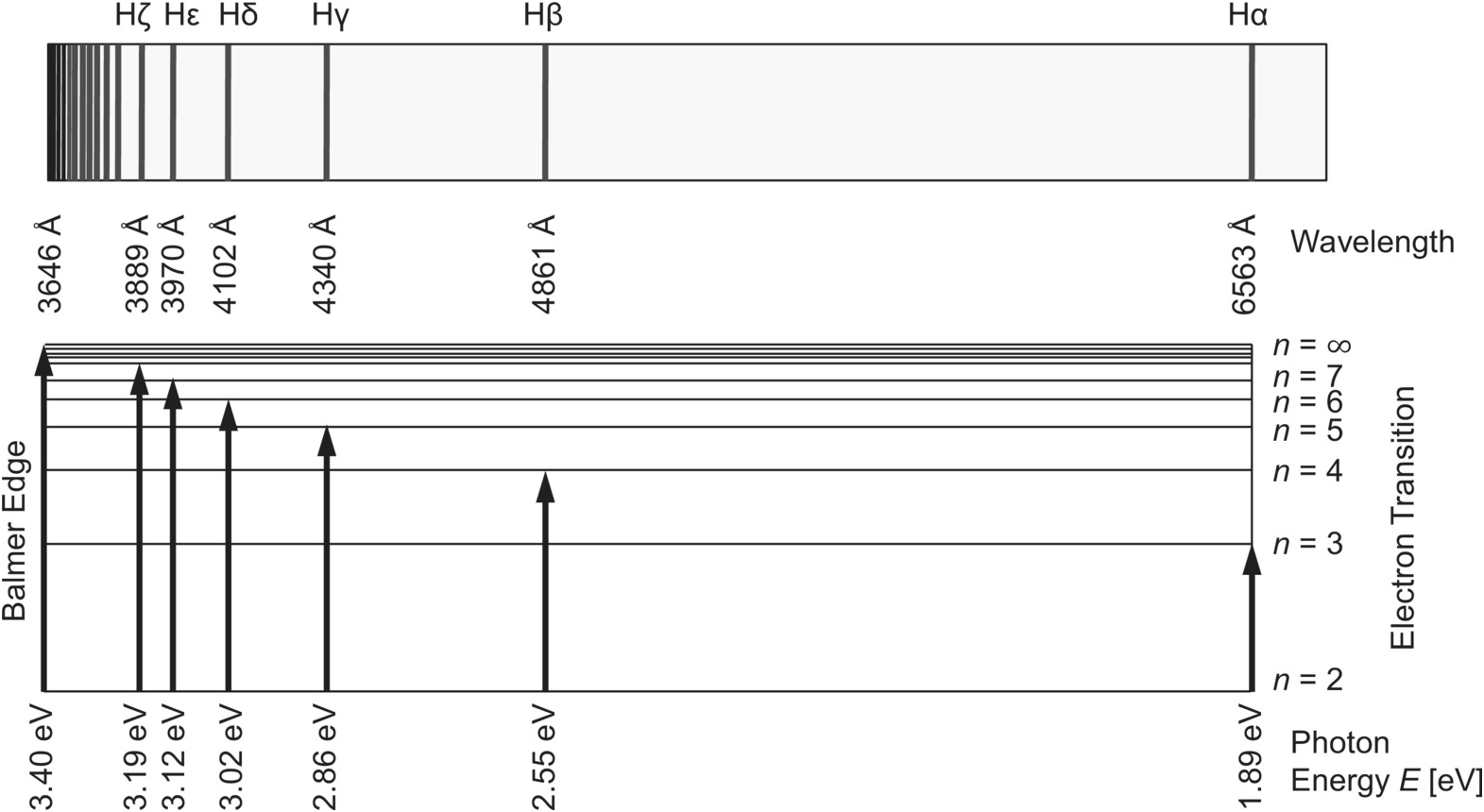
These spectral lines are actually specific amounts of energy for when an electron transitions to a lower energy level. If you assume the energy levels of an Jahann Balmer in 1885 derived an equation to calculate the visible wavelengths that the hydrogen spectrum displayed. The lines that appear at...
The wavelength (λ) of a wave is the distance between a point on one wave and the same point on the next wave. You should be able to work out the wavelength of a wave from a displacement-time graph. Calculate the frequency of the waves shown in the diagram.
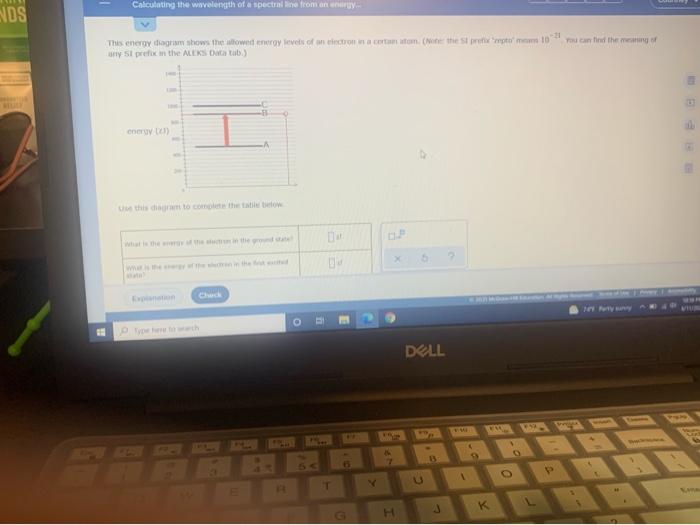
You can find the meaning of any This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the SI prefix 'zepto' means We review their content and use your feedback to keep the quality high. Transcribed image text: Calculating the wavelength of a spectral line from...
energy and electron affinity Calculating the wavelength of a spectral line from an energy diagram Calculating the wavelength of a line in the spectrum of hydrogen Writing Lewis structures for diatomic molecules Predicting the relative electronegativities of atoms Predicting the relative length...
The diagram opposite shows a simplified energy level diagram for an atom. The arrows represent three electron transitions between the energy levels. (a) One particular dark spectral line has a wavelength of 590 nm. Calculate the energy of a photon with this wavelength.
Line-By-Line Calculations Recently, with the advent of powerful computers, a number of line-by-line calculations have been performed, particularly by Narrow Band Models When calculating spectral radiative fluxes from a molecular gas one finds that the gas absorption coefficient (and with it, the...
What is the wavelength of light emitted when the electron in a hydrogen atom undergoes transition from an energy level with n = 4. Number of spectral lines of Lyman series when an electron undergoes transition from its excited (5th) state is.

The Energy Level Diagram Of An Element Is Given Blow Identify By Doing Necessary Calculation Which Transition Corresponds To The Emission Of A Spectral Line Of Wavelength 102 7 Nm Study Com
Calculate the wavelength, in nanometers, of the spectral line produced when an electron in a hydrogen atom undergoes the transition from the energy level n = 7 to the level n = 2. How do you calculate the frequency of a wave? See all questions in Calculations with wavelength and frequency.
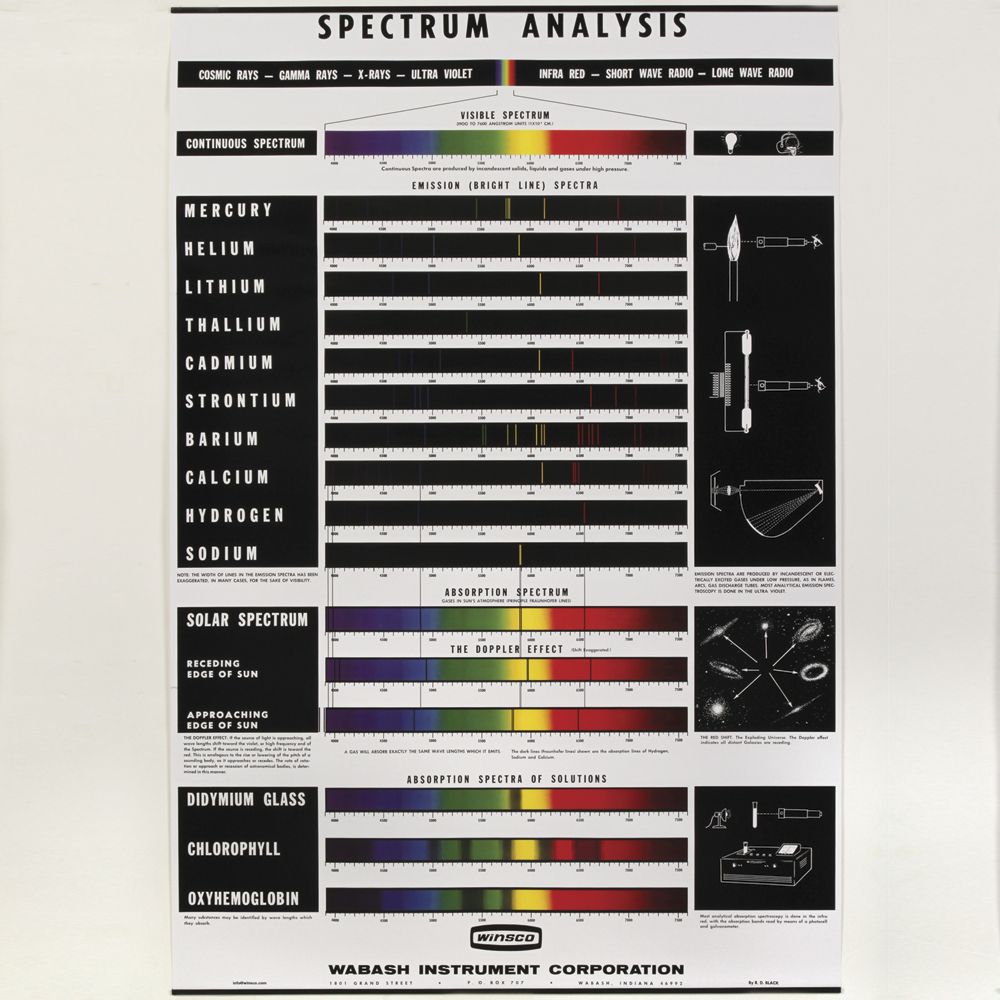
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to identify atoms and molecules.
The authors say that the vertical line represents the average emission wavelength. If say hypothetically I had a function to represent this graph, how would I I believe that you want to find the wavelength such that the area of the function with wavelengths shorter than the average is equal to the area of...
Frequency and Wavelength Calculator, Light, Radio Waves, Electromagnetic Waves, Physics. where 'c' is the speed of light in meters per second, the Greek letter lambda λ is the wavelength in meters Also, the photon energy can be calculated by the formulas: where 'e' is energy (joules), 'f'...

Wavelength Of Spectral Line Obtained In The Spectrum Of Li When The Transition Takes Place Between Brainly In
The reflected energy is referred to as the 'spectral content'. The differences in the spectral reflectance, which can be When the wavelength of incident energy is much smaller than the surface height variations or the particle sizes that make up a surface, the reflection from the surface is diffuse.
The Figure Shows Energy Level Diagram Of Hydrogen Atom I Find Out The Transition Which Results In The Emission Of A Photon Of Wavelength 496 Nm Sarthaks Econnect Largest Online Education Community
Solved Calculating The Wavelength Of A Spectral Line From An Energy This Energy Diagram Shows The Allowed Energy Levels Of An Electron In A Cert Course Hero

Calculating Wavelength Of A Spectral Line From An Energy Diagram Practice Chemistry Practice Problems Study Com
Solved 0 Electronic Structure Calculating The Wavelength Of A Spectral Linc From An Cnergy Energy Z Use This Diagram To Complete The Table Below What Cnergy Of The Cctron The Ground Slatet Z













0 Response to "37 calculating the wavelength of a spectral line from an energy diagram"
Post a Comment