37 a student who is studying atomic reactions creates the following venn diagram.
Start studying Science Unit 4 Lesson 3. Learn vocabulary, terms, and more with flashcards, games, and other study tools. A student who is studying atomic reactions creates the following Venn diagram. Circle 1: Reaction A -Involves Reactions -Happens outside atomic nucleus ...
Choose all the answers that apply. Nitrogen has an atomic number of 7. Its atomic mass is 14. Nitrogen has _____. seven electrons seven neutrons seven … electron shells 23 neutrons 14 electrons Please help (:
A student who is studying atomic reactions creates the following venn diagram.
Q. Christy is studying sodium in the following periodic table of elements. She makes the following list of possible properties for sodium: Symbol is Na . Is a transition metal . Atomic number is 11 . Has one valence electron . Which of these should Christy remove from her list? What is the nuclear binding energy of an atom that has a mass defect of 1.643 mc030-1.jpg 10-28 kg? Use E = mc2. (Remember: The speed of light is approximately 3.00 mc030-2.jpg 108 m/s.) Q. A student determines the density, solubility, and boiling point of two liquids, Liquid 1 and Liquid 2. Then he stirs the two liquids together and heats them. After stirring and heating the liquids, two different liquids form, Liquid 3 and Liquid 4. Then the student determines the density, solubility, and boiling point of Liquids 3 and 4.
A student who is studying atomic reactions creates the following venn diagram.. Correct answers: 1 question: A student who is studying atomic reactions creates the following Venn diagram. Reaction A - Involves electrons -Happens outside atomic nucleus - Releases relatively small amounts of energy. Reaction b - Involves neutrons - Happens inside atomic nucleus - Releases relatively large amounts of energy Question 7. SURVEY. 180 seconds. Q. During an investigation, students dropped a tarnished penny into a solution a acetic acid and sodium chloride. Tiny bubbles formed around the penny, and the penny was less tarnished after two minutes of soaking in the acetic acid and sodium chloride solution. The student inferred that a chemical reaction occured. 29 May 2021 — A student who is studying atomic reactions creates the following Venn diagram. Circle 1: Reaction A -Involves Reactions A student who is studying atomic reactions creates the following Venn diagram. Circle 1: Reaction A -Involves Reactions -Happens outside atomic nucleus - ...2 answers · 0 votes: Answer: A reaction A: chemical; reaction B: nuclear Explanation: edg 2021
A student who is studying atomic reactions creates the following Venn diagram. Reaction A - Involves electrons-Happens outside atomic nucleus - Releases relatively small amounts of energy. Reaction b - Involves neutrons - Happens inside atomic nucleus - Releases relatively large amounts of energy Q: t Activity N Think About It Briefly answer the following question in the space provided Miss Wray has an "oxygen in use" sign posted outside of her room. Why is it e A:See Answer; Q: 2. The binary system of components P and Q conforms closely to Raoult's law. Using the vapor pressure data given below prepare the following: a. A student who is studying atomic reactions creates the following Venn diagram. Circle 1: Reaction A -Involves Reactions-Happens outside atomic nucleus -Releases relatively small amounts of energy Circle 2: Reaction B-Involves neutrons-Happens inside atomic nucleus-Releases relatively large amounts of energy Q. A student determines the density, solubility, and boiling point of two liquids, Liquid 1 and Liquid 2. Then he stirs the two liquids together and heats them. After stirring and heating the liquids, two different liquids form, Liquid 3 and Liquid 4. Then the student determines the density, solubility, and boiling point of Liquids 3 and 4.
What is the nuclear binding energy of an atom that has a mass defect of 1.643 mc030-1.jpg 10-28 kg? Use E = mc2. (Remember: The speed of light is approximately 3.00 mc030-2.jpg 108 m/s.) Q. Christy is studying sodium in the following periodic table of elements. She makes the following list of possible properties for sodium: Symbol is Na . Is a transition metal . Atomic number is 11 . Has one valence electron . Which of these should Christy remove from her list?

Dietary Manganese Requirement And Its Effects On Antioxidant Enzyme Activities Intestinal Morphology And Microbiota In Oriental River Prawn Macrobrachium Nipponense De Haan Sciencedirect

A New Analysis Of Mars Special Regions Findings Of The Second Mepag Special Regions Science Analysis Group Sr Sag2 Astrobiology
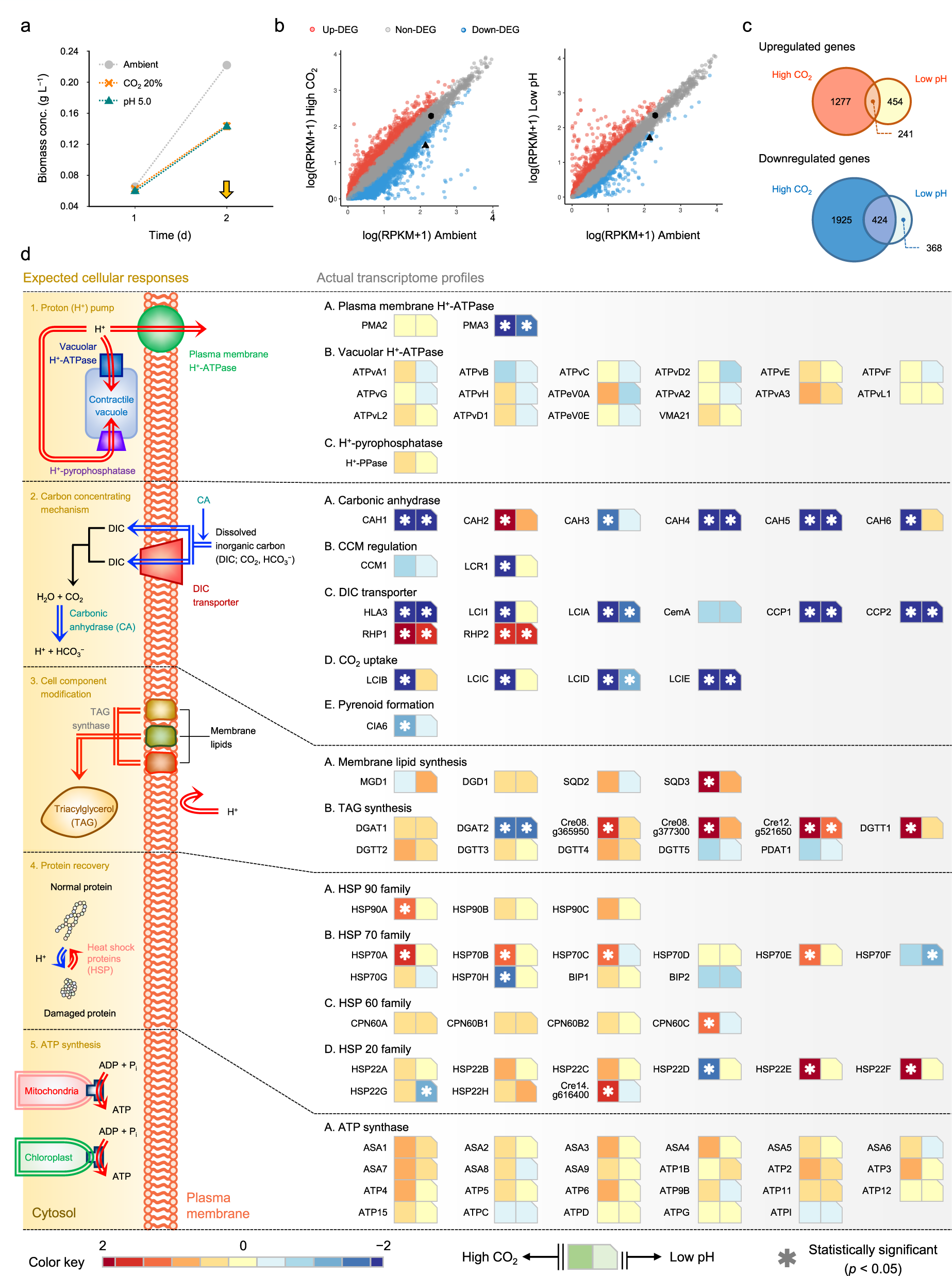
Augmented Co2 Tolerance By Expressing A Single H Pump Enables Microalgal Valorization Of Industrial Flue Gas Nature Communications

Pdf The Effectiveness Of Using Digital Game Towards Students Academic Achievement In Small And Large Classes A Comparative Research

Rhode Island Department Of Education Instruction Assessment Curriculum Curriculum Frameworks Science Curriculum Frameworks
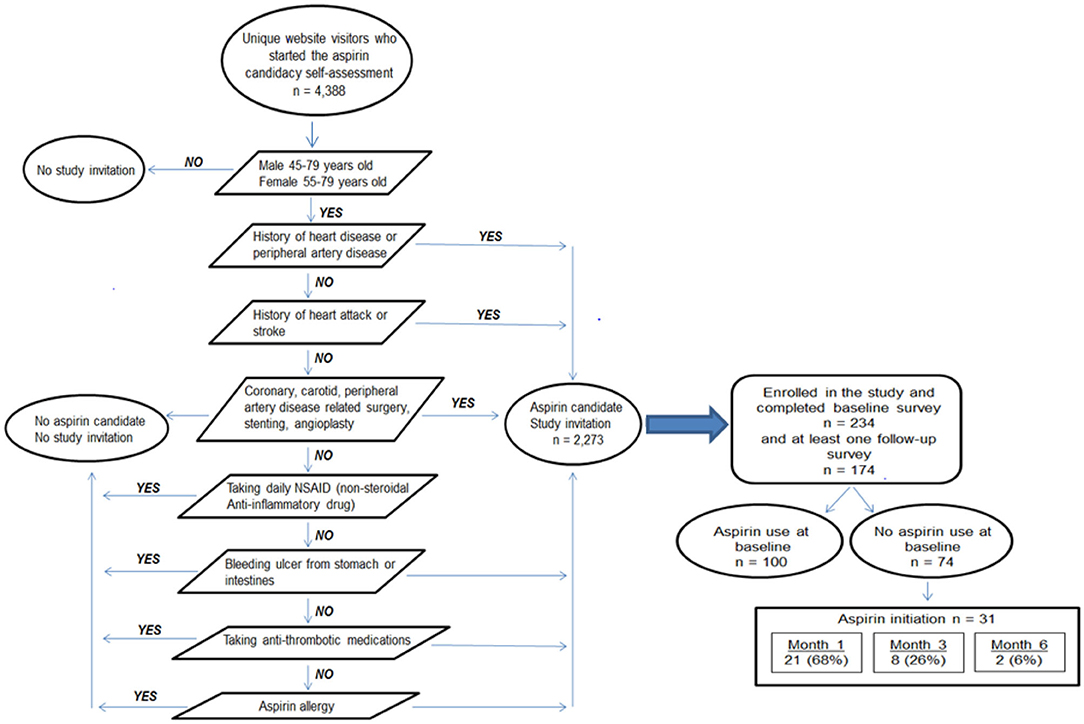
Https Www Frontiersin Org Articles 10 3389 Fpubh 2021 641754 Https Www Frontiersin Org Files Articles 641754 Fpubh 09 641754 Html Image M Fpubh 09 641754 T005 Jpg Table 5 Participant Responses On Feasibility And Utility Survey Https Www Frontiersin

A Student Who Is Studying Atomic Reactions Creates The Following Venn Diagram Reaction A Involves Brainly Com

Pdf Variability In The Chemistry Of Private Drinking Water Supplies And The Impact Of Domestic Treatment Systems On Water Quality


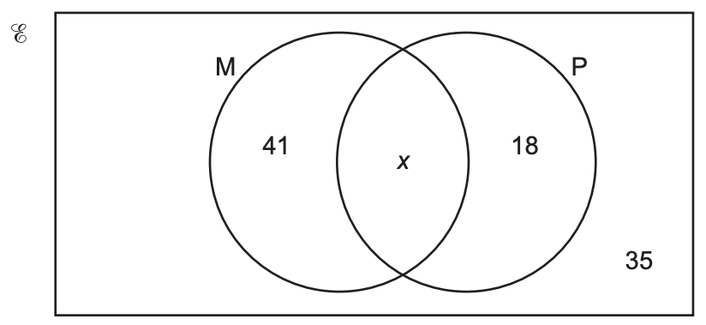

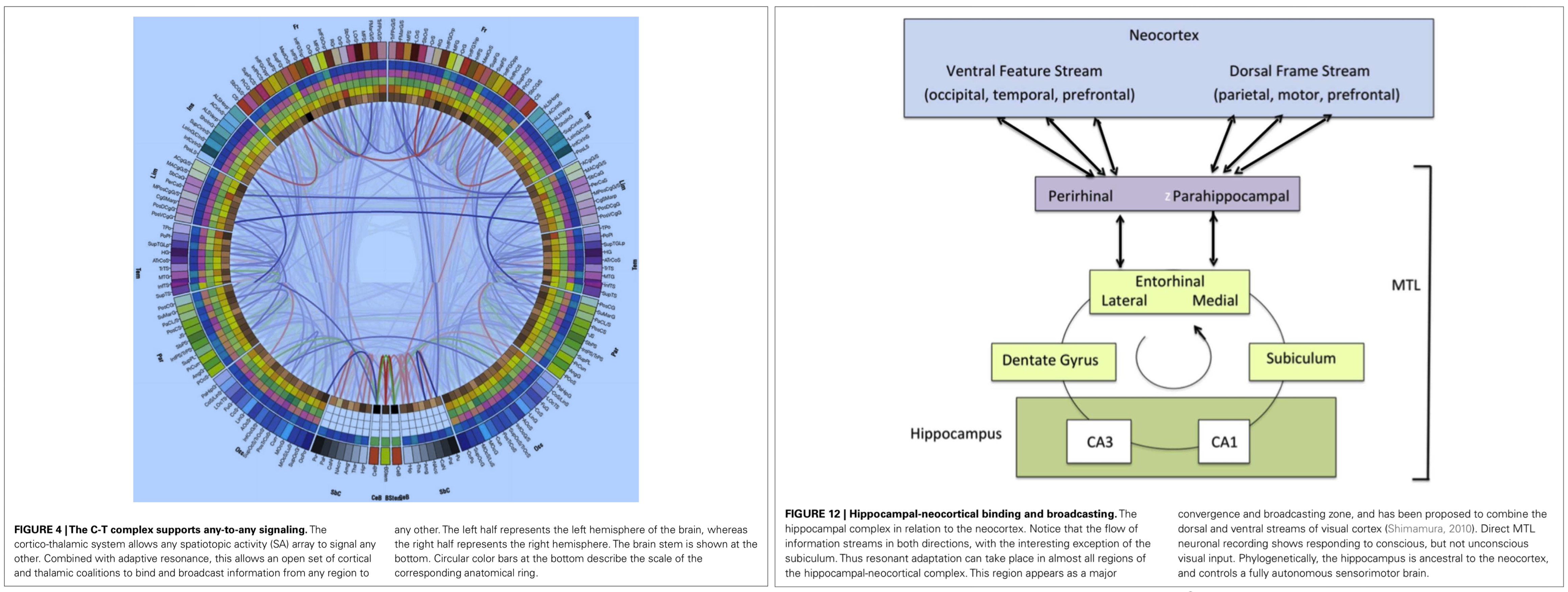









0 Response to "37 a student who is studying atomic reactions creates the following venn diagram."
Post a Comment