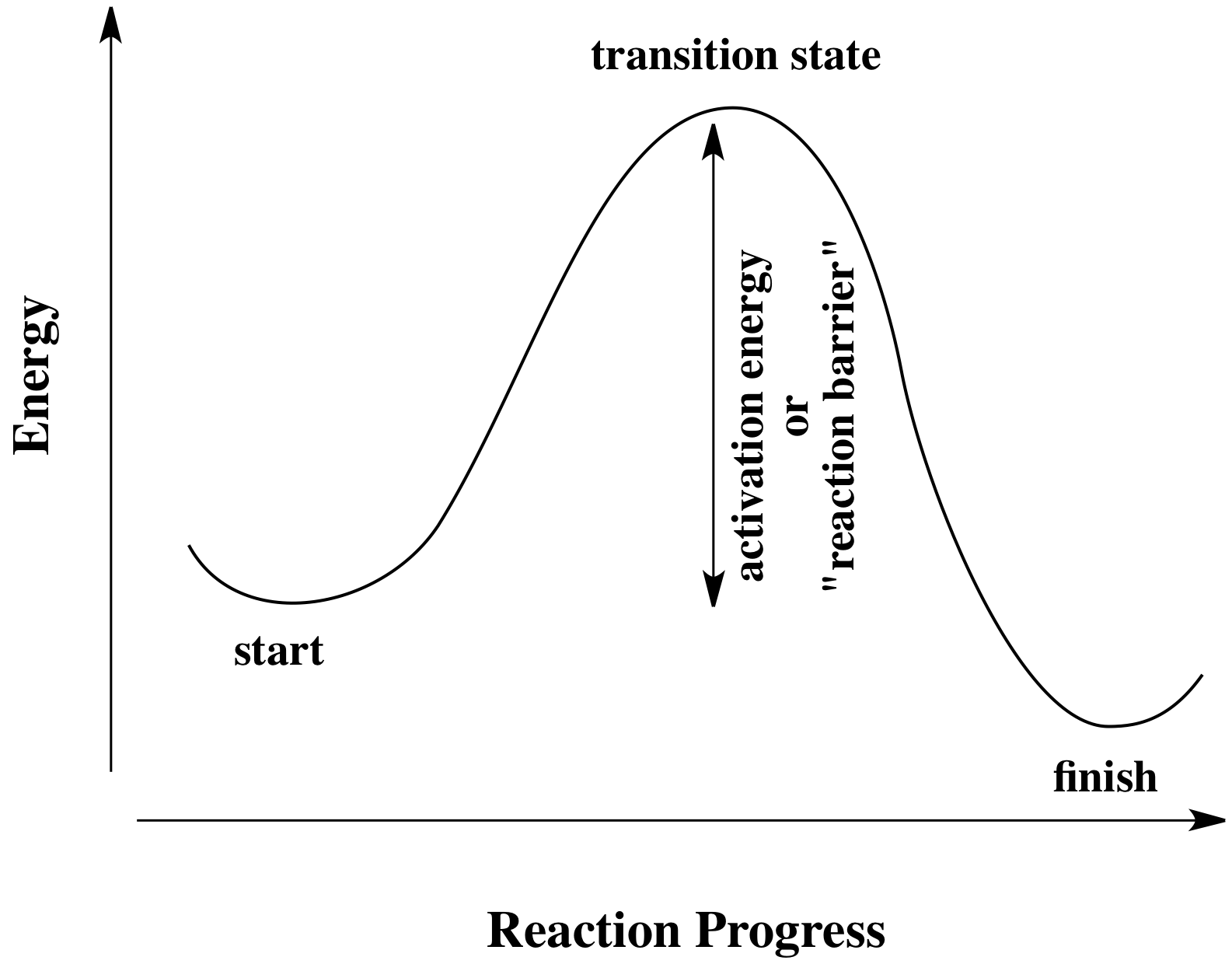
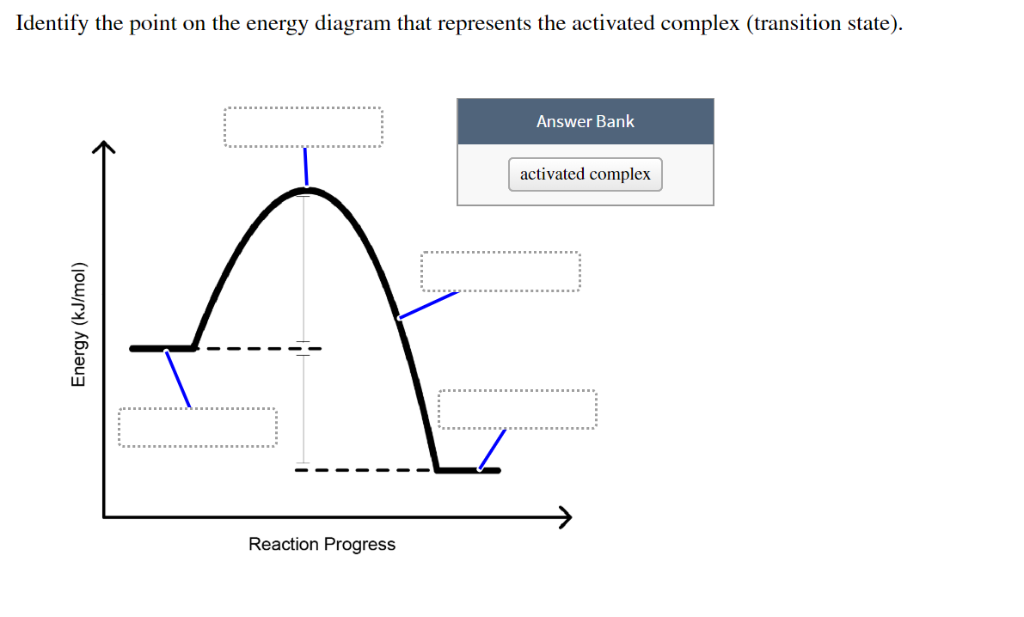
39 click on the point of the energy diagram that represents the activated complex (transition state).
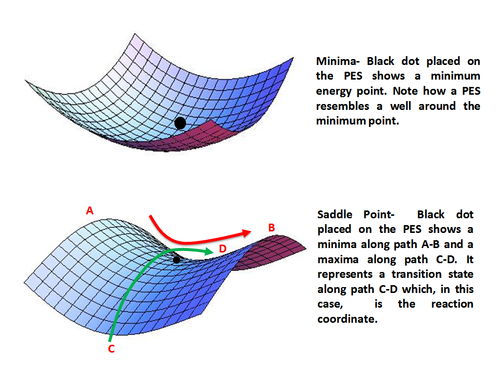
transition-state theory, also called activated-complex theory or theory of absolute reaction rates, treatment of chemical reactions and other processes that regards them as proceeding by a continuous change in the relative positions and potential energies of the constituent atoms and molecules.On the reaction path between the initial and final arrangements of atoms or molecules, there exists ... This is part 3 of a four part series in the Energy Diagram Module. Stay tuned for Part 4! Click on the following links to see earlier parts: Part 1. Part 2. Sometimes reactions are more complex than simply a transition state (Graph 3), which would represent a single step in the reaction mechanism.
Activation energy (Ea) is the difference between the potential energy of intermediate "activated complex/ peak" and reactants. Ea = P.E. of products - P.E. of reactants = 65 kJ - 30 kJ = 35 kJ. kindly find the attached image.
Click on the point of the energy diagram that represents the activated complex (transition state).
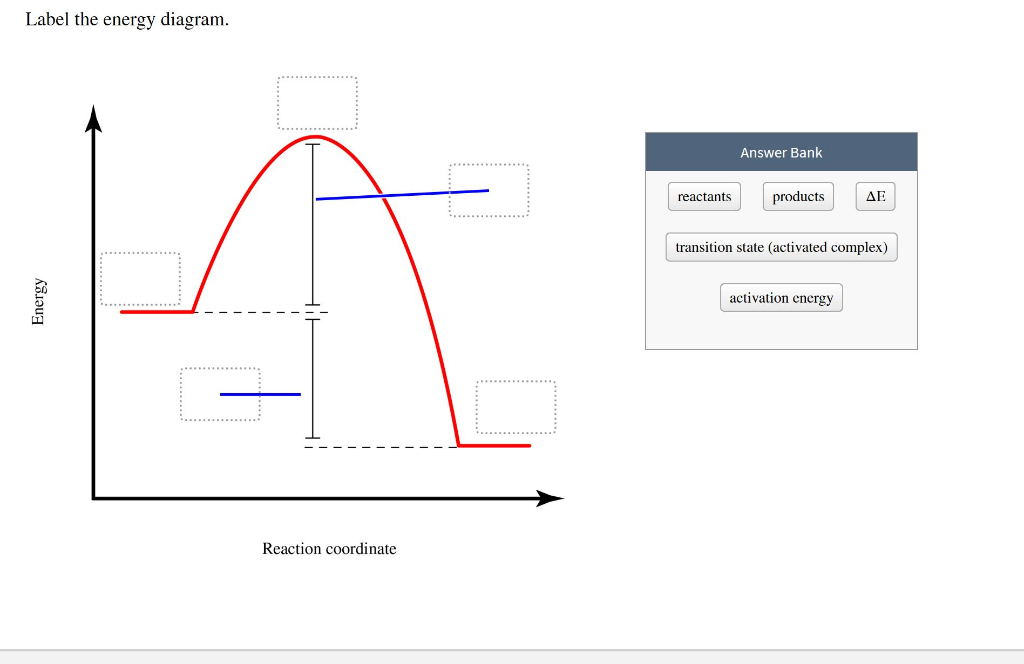
Transcribed Image Textfrom this Question. Label this diagram. *Note that a transition state is also known as an activated complex . Which curve represents the catalyzed reaction? blue (top) green (bottom) The figure shows that the energy of the transition state is higher than that of the reactants A and B by an amount equal to E a, the activation energy.Thus, the sum of the kinetic energies of A and B must be equal to or greater than E a to reach the transition state. After the transition state has been reached, and as C and D begin to form, the system loses energy until its total energy is ... So this is Ea. That represents our activation energy, which for this reaction would be 80 minus 20, which would be equal to 60 kilojoules per mol. So we get to here. We get to the transition state or the activated complex, and then this would represent the energy of our products. So this our energy of our products here, which for this reaction ...
Click on the point of the energy diagram that represents the activated complex (transition state).. At the very top of the energy barrier, the reaction is at its transition state (TS), which is the point at which the bonds are in the process of breaking and forming. The transition state is an ' activated complex' : a transient and dynamic state that, unlike more stable species, does not have any definable lifetime. Reaction coordinate diagrams clearly show that the energy of an enzyme bound to a transition state is higher than the energies of the E + S, E + P, and ES that occur along the same reaction coordinate. The energy of an enzyme bound to a transition state analog would lie _____ in the diagram. a. above the E + S but below the transition state Transition state theory suggests that as molecules collide and a reaction takes place, they are momentarily in a strained or less stable state than either the reactants or the products. During this transition state, the potential energy of the activated complex increases, effectively creating an energy barrier between the reactants and The activated complex- as resembled by the peak between the reactants and the products- however, possesses the most significant amount of chemical potential energy among all three states since by definition the complex (intermediate state) can spontaneously proceed either forward to the products or backward to the products without any ...
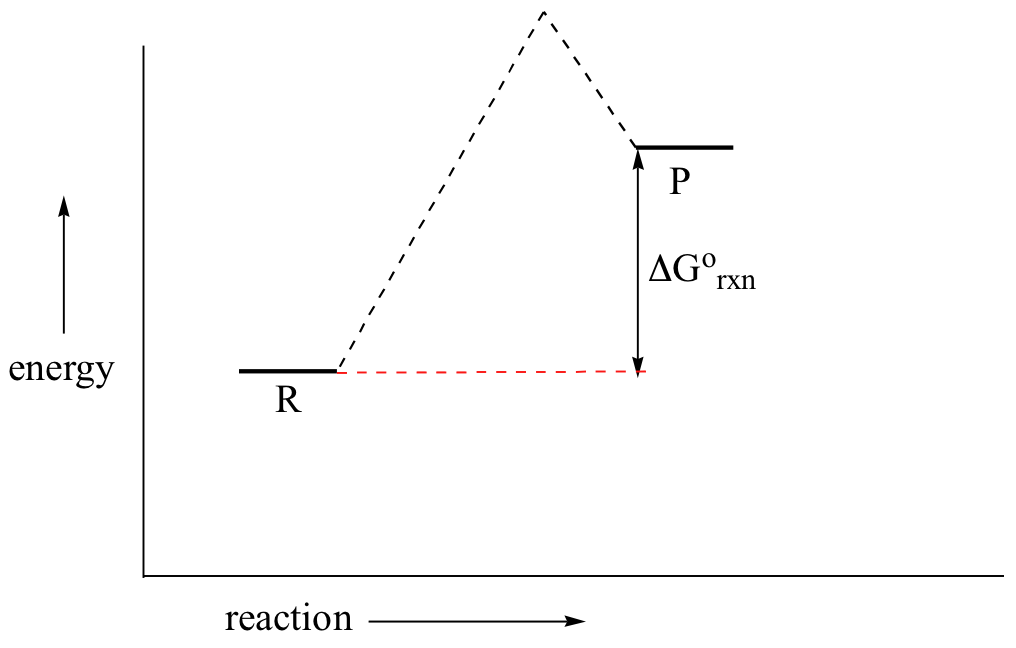
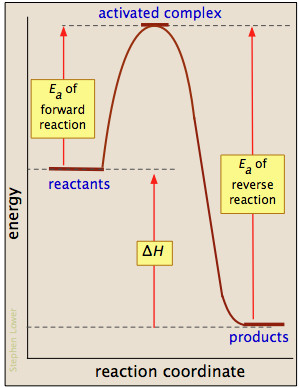
between the reactant energy and the transition state at the peak of the diagram. The activation energy for the reverse reaction, E a(rev), is the difference between the product energy and transition state at the peak of the diagram. ΔH r is the difference between the potential energy of the reactant and the potential energy of the product. a. In transition state theory (TST) an activated molecule is formed during the reaction at the transition state between forming products from reactants. The rate of reaction is equal to the product of the frequency, v I, of the activated complex crossing the barrier and the concentration of the transition state complex. In this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled. At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products. This state is also known as an activated complex. An activated complex is an unstable state that is between the reactants and the products in a chemical reaction. During the transition state, molecules collide and break bonds. The activated ...
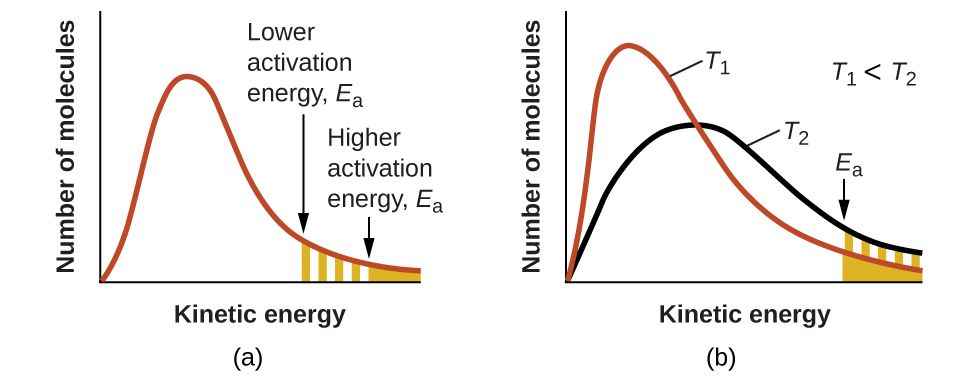
These reaction diagrams are widely used in chemical kinetics to illustrate various properties of the reaction of interest. Viewing the diagram from left to right, the system initially comprises reactants only, A + B. Reactant molecules with sufficient energy can collide to form a high-energy activated complex or transition state. The activation energy is the difference between the energy of the reactants and the maximum energy (i.e. the energy of the activated complex). The reaction between H 2 ( g) and F 2 ( g) ( Figure 12.4) needs energy in order to proceed, and this is the activation energy. To form the product the bond between H and H in H 2 must break. This indicates the use of a catalyst in diagram (b). The activation energy is the difference between the energy of the starting reagents and the transition state—a maximum on the reaction coordinate diagram. The reagents are at 6 kJ and the transition state is at 20 kJ, so the activation energy can be calculated as follows: Transition state theory (TST), also called activated complex theory, is often introduced in general chemistry courses when discussing kinetics. A reaction energy diagram is used to follow the progress of the reaction from reactants through a transition state to products (see figure 1). The reaction energy diagram plots the
on the energy diagram that represents the activated complex (transition state) Identify the point Answer Bank activated complex Reaction Progress Energy (kJ/mol) Question: on the energy diagram that represents the activated complex (transition state) Identify the point Answer Bank activated complex Reaction Progress Energy (kJ/mol)
The state represented by the double dagger symbol is known as the transition state and represents the exact configuration that has an equal probability of forming either the reactants or products of the given reaction. The activated complex is often confused with the transition state and is used interchangeably in many textbooks.
Activated Complex. When reactant molecules collide with each other at its highest energy point, an intermediate is formed which remains in equilibrium with the main reactant. If this intermediate complex has an energy equal to or greater than the Threshold Energy then it will be converted into a product. Activation Energy Formula
Click on the point of the energy diagram that represents the activated complex transition state. The transition state is an activated complex. At the very top of the energy barrier the reaction is at its transition state ts which is the point at which the bonds are in the process of breaking and forming.

Activation Energy E A Is The Minimum Energy That Reactants Must Have To Form Products The Height Of The Potential Barrier Sometimes Called The Energy Ppt Download
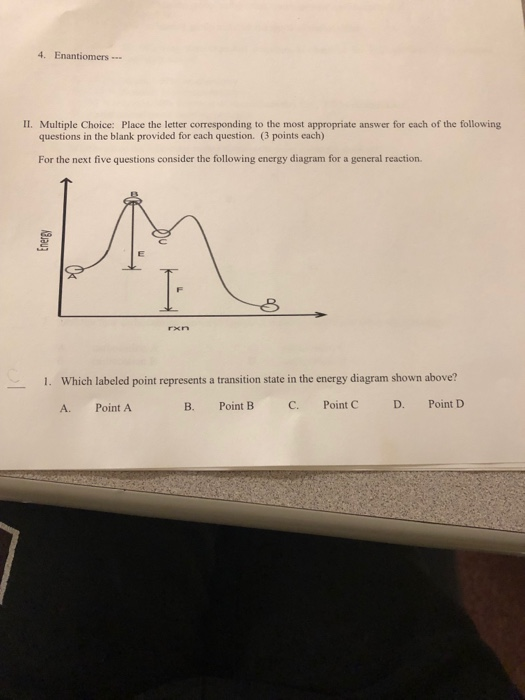
Answer : The letter represents the activation energy for the reverse reaction is, U. Explanation : Activation energy : It is defined as the minimum amount of energy given to the reactant so that it gets converted into products. Or we can say that, It is the minimum amount of energy that is absorbed by the reactant molecules so that their energy becomes equal to the threshold energy.
At the very top of the energy barrier, the reaction is at its transition state (TS), which is the point at which the bonds are in the process of breaking and forming. The transition state is an ' activated complex' : a transient and dynamic state that, unlike more stable species, does not have any definable lifetime.
The activated complex covers a range of atom configurations that atoms form on their way from reactant to products. In other words, the transition state is the one molecular configuration that occurs at the peak of the energy diagram of the reaction. The activated complex may be present at any point near the transition state.

3 Please Answer The Following Questions Concerning The Two Energy Diagrams Depicted Below 10 Pts Energy Homeworklib
The second diagram where the bonds are half-made and half-broken is called the transition state, and it is at this point that the energy of the system is at its maximum.This is what is at the top of the activation energy barrier. But the transition state is entirely unstable.
• The activation energy is needed because existing bonds must be broken and new bonds must be formed. The point in the reaction at which this reconstruction is occurring is called the transition state or activated complex.
The activation energy is represented with the letter A The potential energy diagram above gives the relative energy of the reactants and the products in a reaction. The x-axis represents the pathway of the reaction, so in this general case, we have: reactants ->products The particles that are the reactants in this reaction have a certain amount of potential energy, which is in this case 100 (y ...
The highest point in a reaction coordinate diagram represents _____. A) an intermediate of the reaction pathway B) the reactants in an exergonic reaction C) the products in an endergonic reaction D) the transition state E) the overall G for the reaction
So this is Ea. That represents our activation energy, which for this reaction would be 80 minus 20, which would be equal to 60 kilojoules per mol. So we get to here. We get to the transition state or the activated complex, and then this would represent the energy of our products. So this our energy of our products here, which for this reaction ...
The figure shows that the energy of the transition state is higher than that of the reactants A and B by an amount equal to E a, the activation energy.Thus, the sum of the kinetic energies of A and B must be equal to or greater than E a to reach the transition state. After the transition state has been reached, and as C and D begin to form, the system loses energy until its total energy is ...
Transcribed Image Textfrom this Question. Label this diagram. *Note that a transition state is also known as an activated complex . Which curve represents the catalyzed reaction? blue (top) green (bottom)

Chemical Kinetics In Kinetics We Study The Rate At Which A Chemical Process Occurs Besides Information About The Speed At Which Reactions Occur Kinetics Ppt Download

Draw An Energy Diagram For An Exothermic Reaction Label The Activation Enthalpy And The Change In Enthalpy Delta H On The Diagram Study Com
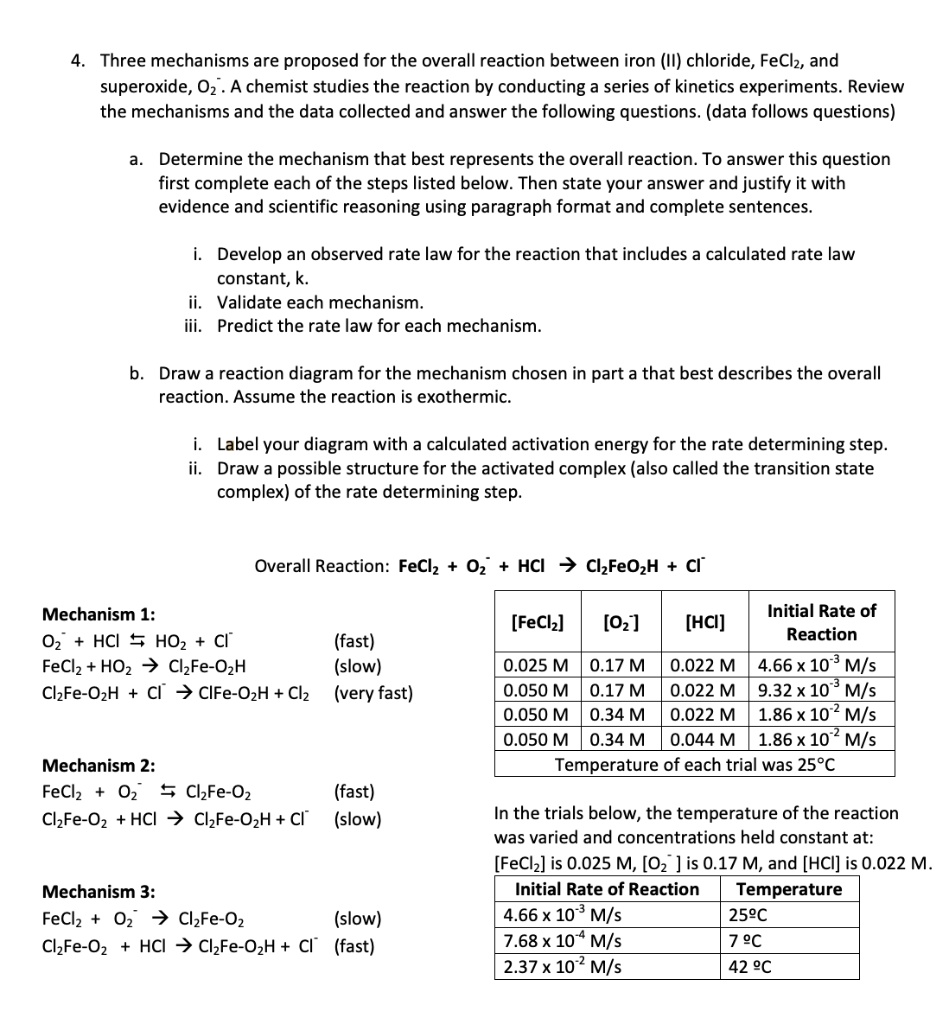
Solved Three Mechanisms Are Proposed For The Overall Reaction Between Iron Ii Chloride Feclz And Superoxide 02 A Chemist Studies The Reaction By Conducting A Series Of Kinetics Experiments Review The Mechanisms And











0 Response to "39 click on the point of the energy diagram that represents the activated complex (transition state)."
Post a Comment