39 electron dot diagram for iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 degrees Celsius, and boils to a violet gas at 184 degrees Celsius.The element was discovered by the French chemist Bernard Courtois in 1811, and was named two years later ... Electronic Devices And Circuits Jacob Millman Pdf, New Electronic, Electronic Devices And Circuits Jacob Millman Pdf
chemistry. Identify the neutral element represented by this excited-state electron configuration, then write the ground-state electron configuration for that element.
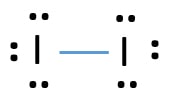
Electron dot diagram for iodine
Hence, the Lewis structure of iodine trichloride would be: We can observe that every chlorine atom is surrounded by eight electrons but a central atom, iodine, is surrounded by ten electrons. It is one of the exceptions of the octet rule, i.e., the elements of the third period or beyond the third period of the periodic table have 3d electrons ... (e) Draw the electron dot structure for the formation of this oxide. Answer. Question.11. An element X (atomic number 17) reacts with an element Y (atomic number 20) to form a divalent halide. (a) Where in the periodic table are elements X and Y placed? (b) Classify X and Y as metal(s), non- metal(s) or metalloid(s). Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard-brittle, grayish-white metalloid in the carbon group, chemically similar to its group neighbors silicon and tin.Pure germanium is a semiconductor with an appearance similar to elemental silicon. Like silicon, germanium naturally reacts and forms complexes with oxygen in nature.
Electron dot diagram for iodine. I3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. I3- or triiodide ion is a polyatomic molecule or a charged molecule having a net negative charge of -1. I2 + I- —-> I3-. This is the exergonic equilibrium leading to the formation of the ion where a positive flow of energy happens from the system to the surroundings. Statistics and posts of IIT JEE NEET BITSAT Books Notes ™ telegram channel. Telegram's Best Study Channel, Providing Content for NEET, JEE & BITSAT Exams. Handled By IITians and AIIMSonians. Get Our Notes & Tips for Free Here. Keep Sharing & Supporting Us.. Subscriber gain, reaches, views iit_jee_neet_bitsat_books_notes on Telemetrio. 📚 HALIDES - [CHEMISTRY] 📝 SHORT HANDWRITTEN NOTES ... Draw (on paper) a Lewis structure for IFs and answer the following questions based on your drawing. 1. For the central iodine. 1 answer Salle CH) rect to MO A volte call similar to that shown in the figure above is constructed. The electronic device shown at the top of the figures a volte One Hectrode compartment of a countrie placed Q.draw a lewis structure for a compound with molecular formula c4h11n in which three of the carbon atoms are bonded to the nitrogen atom . This is also called a lewis structure. Atom epg molecular shape ci fcl for the iodine atom epg molecular shape. It is defined as the it shows the bonding between the atoms of a molecule and it also shows the ...
Lawn boy parts diagram Beverly Jarosz, a sweet tempered 16 yr old high school junior, lived in Garfield Hts Ohio with her parents Thaddeus and Eleanor and her 12 yr old sister, Carol. She was a quiet girl who friends said was a touch moody and secretive, yet a warm friend once she trusted you. 7s 2 Electron Dot Model. Diagram of the nuclear composition electron configuration chemical data and valence outer electron orbitals of an atom of radium-226 atomic number. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 2 6p 6 7s 2. Number of Neutrons most commonstable nuclide. Two separate fluorine atoms have the following electron dot diagrams: Each fluorine atom contributes one valence electron, making a single bond and giving each atom a complete valence shell, which fulfills the octet rule: The circles show that each fluorine atom has eight electrons around it. As with hydrogen, we can represent the fluorine ... Lewis Structure is one of the key terminologies to understand the chemical bonding of a molecule since it represents the molecular structure. It depends on the octet rule concept and is an extension of the electron dot diagram. Thus, to have a comprehensive idea about CS2 Lewis Structure, let us go through each step clearly and systematically.
One of the easiest methods is the iodine-thiosulfate titration therapy. Iodide ion, I-, is shortly oxidized by almost any oxidizing agent (It has plenty of electrons to lose!). In an acid resolution, hypochlorite ions oxidize iodide ions to type iodine, I2. The iodine that forms is then titrated with a standard service of sodium thiosulfate. While the Lewis Structure provides an idea about the physical attributes of the compound, its representation is limited since it is a 2-dimensional model. It also does not reflect upon the molecular design, geometry, or the 3-dimensional representation of atoms. below are the steps to draw the lewis diagram of the Br2 molecule. Methane (US: / ˈ m ɛ θ eɪ n /, UK: / ˈ m iː θ eɪ n /) is a chemical compound with the chemical formula CH 4 (one atom of carbon and four atoms of hydrogen).It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas.The relative abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it poses technical challenges ... Potassium and iodine d. Calcium and nitrogen. O ba cl al ar ca n k i ionic bonding worksheet page 2. Number of valance electrons. Ionic bonding lewis dot. Zinc or aluminum if h 2 gas tank is not available 4 6m hcl if h 2 gas tank is not available. ... Electron Dot Diagram Worksheet Chemistry Worksheets Ionic Bonding Electrons .
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal.The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer (passivation) somewhat stabilizes the free metal against further oxidation.. Andrés Manuel del Río discovered compounds of vanadium in 1801 in Mexico by ...
The lewis structure of KCN is: In potassium cyanide, potassium has only one valence electron, carbon has six valence electrons, and nitrogen has seven valence electrons. In the lewis structure of the potassium cyanide, there are two covalent bonds, one coordinate bond, and one ionic bond present. Is agno2 ionic or covalent?
Q. This is a correct dot diagram for neon, group 18. Q. This could be the dot diagram of. Mg, group 2. Cl, group 17. Magnesium lewis dot diagram. It is MgBr2. Magnesium has an electronic configuration of 2.8. 2. It has to lose 2 electrons to attain a stable electronic configuration and octet valance shell structure resembling that of noble gases.
In electron dot structure, the valence shell electrons are represented by crosses or dots. (a) The atomic number of chlorine is 17. Write its electronic configuration (b) Draw the electron dot structure of chlorine molecule. Answer: (a) 2, 8, 7. Question 42. Catenation is the ability of an atom to form bonds with other atoms of the same element.
Diluted iodine soap ie. In lab groups split the 4 tests amongst the lab group members. This cell structure and function worksheet answer key maybe his advice merited some attention. Water oil milk oatmeal apple juice and Unknown X. Macromolecules In Food Lab Answer Key. ...
Lewis Structure of PCl5. Lewis structure of a compound is the arrangement of its underlying atom's valence shell electrons. Lewis structures make the use of dots to represent electrons and bonds between different electrons are represented through a straight line, marked at the end of which is a set of electrons.
NCl3 from the air environment reacts with DPD 3 releasing iodine which reacts with DPD 1 and produces a coloration proportional to the amount of NCl3 from the sampled indoor swimming pool air. I know the NCl3 has a pyramidal shapeBut is N-Cl a polar bond. Yes it is polar like PCl3 AsCl3 or NCl3.
Sulfur (in nontechnical British English: sulphur) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic.Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S 8.Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most common element by mass in the universe ...
Electron Transfer. We can use electron configurations to illustrate the electron transfer process between sodium atoms and chlorine atoms. Na: 1s 2 2s 2 2p 6 3s 1. As demonstrated here, a sodium atom (Na) has one valence electron in the third principal energy level.
Since an electron dot structure surrounds an elemental symbol with one dot for every valence electron that the element contains, sulfur's elemental symbol must be surrounded by 6 dots. Count the number of valence electrons when electronic configuration is given: Show the electron shell including the s, p, and d orbitals together.
41 2005 pontiac vibe serpentine belt diagram Written By Stephan T. Hawkins. Monday, November 22, 2021 Add Comment Edit. Replacing the belt tensioner in a 2006 Pontiac Vibe, also same as Toyota Matrix. A little tricky but do able. See part 2 for Motor Mount removal and instal...
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard-brittle, grayish-white metalloid in the carbon group, chemically similar to its group neighbors silicon and tin.Pure germanium is a semiconductor with an appearance similar to elemental silicon. Like silicon, germanium naturally reacts and forms complexes with oxygen in nature.
(e) Draw the electron dot structure for the formation of this oxide. Answer. Question.11. An element X (atomic number 17) reacts with an element Y (atomic number 20) to form a divalent halide. (a) Where in the periodic table are elements X and Y placed? (b) Classify X and Y as metal(s), non- metal(s) or metalloid(s).
Hence, the Lewis structure of iodine trichloride would be: We can observe that every chlorine atom is surrounded by eight electrons but a central atom, iodine, is surrounded by ten electrons. It is one of the exceptions of the octet rule, i.e., the elements of the third period or beyond the third period of the periodic table have 3d electrons ...




0 Response to "39 electron dot diagram for iodine"
Post a Comment