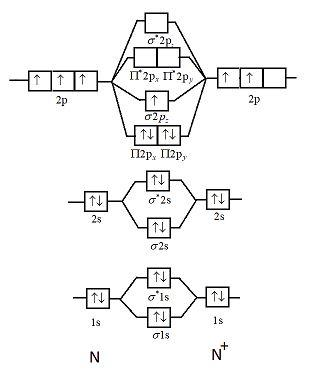
33 n2+ molecular orbital diagram
Place the following molecular orbitals in order of decreasing energy for species of B2, C2, and N2. start with the highest energy orbital. σ₂p* π₂p* σ₂p π₂p. Match each term with the appropriate electron arrangement paramagnetic diamagnetic. A molecular species with one or more unpaired electrons in an MO is _____ and will be attracted to a magnetic field, whereas a species with no ...
21.11.2018 · We assume that orbital order is the same as that for N2. The bond order is Figure The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The ...
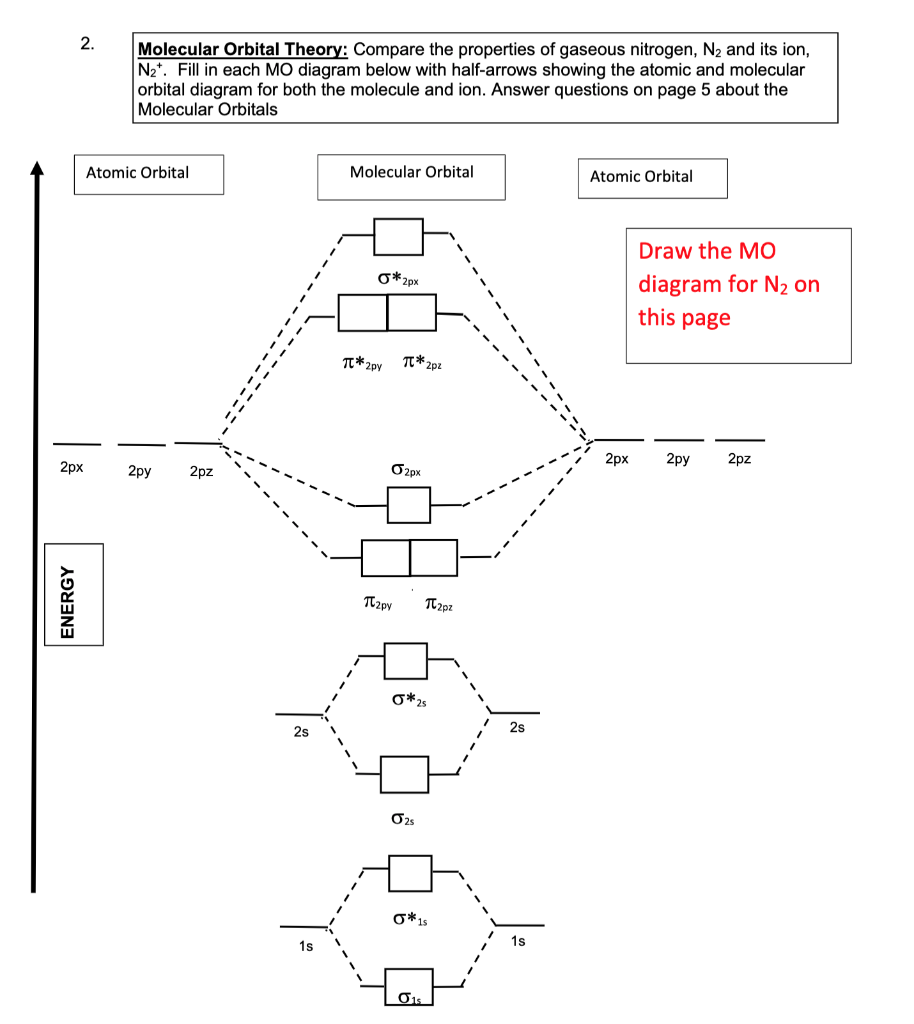
There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start...

N2+ molecular orbital diagram
Molecular orbital diagram for N2 shows that it is diamagnetic. Refer to the MO Diagrams. Cation: Ion with a positive charge. Our crossword puzzle maker allows you Slideshow - Electron configuration. So the qualitative picture of σ and πbonding and antibonding orbitals that we developed for a diatomic like CO can be carried over give a qualitative Molecular Orbital Theory Study how ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) N2 Molecular Orbital Diagram. N2 molecular orbital energy level diagram picture, is usually depicted by a diatomic molecules ...
2 answersLet me explain the molecular orbital diagram of N2 using its diagram. · one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons · so first 2 ...
N2+ molecular orbital diagram.
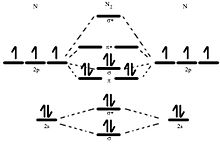
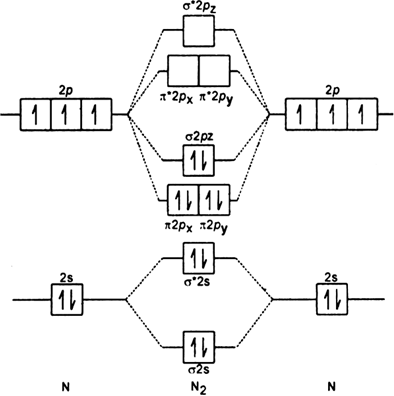
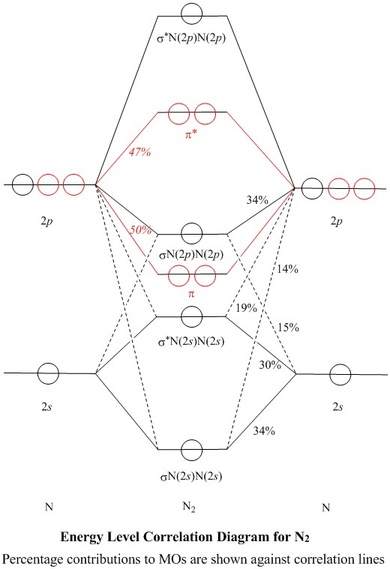
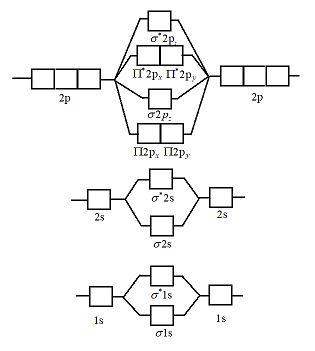
What is the MO diagram and bond order for N2 ( in Urdu / Hindi) Nitrogen (N 2) molecule: Nitrogen atom has electronic configuration 1s2, 2s2, 2p3. Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px.
12+ N2 Molecular Orbital Diagram. One atom of nitrogen has 7 electrons so a n2 molecule will have 14 electrons so first 2 electrons go in 1s sigma bond next 2 in 1s sigma anti bond orbital next 2 in 2s sigma bon. Number of electrons in c2 molecule = 12. Now note that even in this advanced molecular orbital theory a bunch of approximations is ...
the diagram above is the molecular.n2 molecular orbital energy level diagram picture, is usually depicted by a diatomic molecules chapter learn consider the molecular orbital electron configuration notation to a molecular orbitals diagrams web the molecular orbital energy level structures can construct the molecular orbital energy level the …
Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
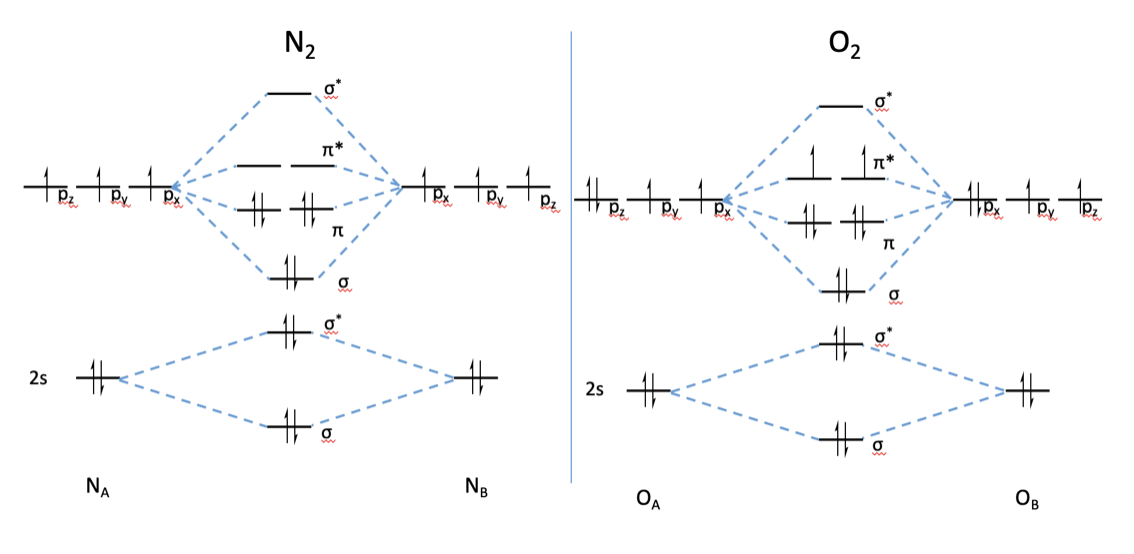
Once Molecular orbital practice problems Ch301 fall 2009 work 7 answer key Molecular Orbital Theory Purdue University April 17th, 2019 - Valence Bond Model vs Molecular Orbital Theory Construct a molecular orbital diagram for the O 2 molecule Click here to check your answer to Practice Problem 9 Bond Order The number of bonds between a pair of atoms Electron configuration and orbital diagram ...
molecular orbital mo diagram of n2 molecular orbital diagram for nitrogen gas n2 use aufbau and hund to fill with 10 valence electrons you sigma2s 2 sigma2s 2 pi2p 4 mo diagram for n2 molecular orbital there are two mo diagrams you need to memorize for diatoms n2 o2 ne2 etc e is for the elements up to nitrogen the other is for after
Molecular orbital theory is a method for describing the electronic structure of the molecule. Now, let us draw the molecular orbital diagram of ${N_2}$ . Now, first let us understand what magnetic behavior and bond order means.
Molecular orbitals in Nitrogen. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. There are four molecular orbitals derived from the 1s and 2s orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The p orbitals combine to produce a sigma and two ...
17.11.2021 · Molecular Orbital Diagram of N2. Molecular orbitals exist in molecules where each molecule has its electron configuration in terms of a sigma bond and pi bond. According to molecular orbital theory, it tells about magnetic nature, stability order, and the number of bonds in a molecule. When two orbitals are added, the result is stable bonding molecular orbital and when orbitals are …
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
20.03.2019 · Energy level diagram for Molecular orbitals. The first ten molecular orbitals may be arranged in order of energy as follow: σ(1s) <σ ∗ (1s) < σ(2s) <σ ∗ (2s) < π(2p x) = π(2p y) < σ(2p z) < π ∗ (2p x) =π ∗ (2p y) <π ∗ ( 2p z) Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons ...
17.10.2018 · Molecular orbital (MO) diagram for N2 and N2^- $-$\mathrm{p}$ interaction moving from $\ce{Li2}$ to $\ce{F2}$. The $\mathrm{s}$-$\mathrm{p}$ interaction is the bonding interaction between the $\mathrm{2s}$ orbital of one atom and the $\mathrm{2p_{z}}$ orbital of another atom which (among other things) increases the energy of the $\mathrm.Molecular orbitals of diatomic …
N2 is a very stable 10-valence-electron molecule, isoelectronic with CO and with [CN] · The formal bond order of N2 is 3, from about one σ-bond and two π-bonds ...
















![Best Answer] draw the molecular orbital diagram of N2 and ...](https://hi-static.z-dn.net/files/d20/b492acf8cb9ff01954c3929a3b7a93c7.jpg)

0 Response to "33 n2+ molecular orbital diagram"
Post a Comment