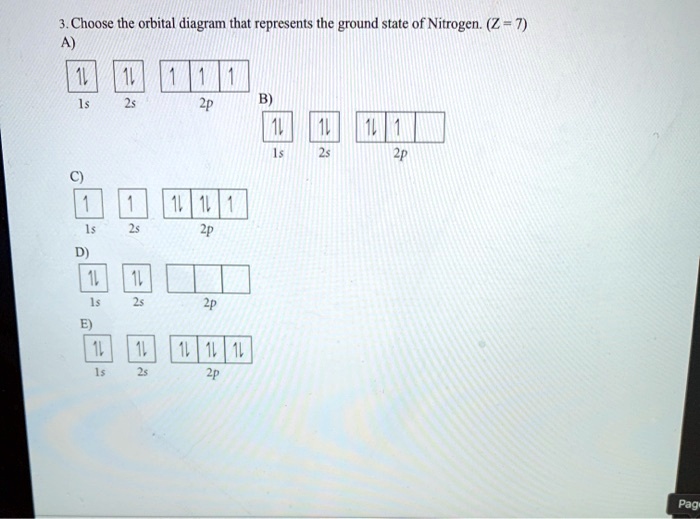
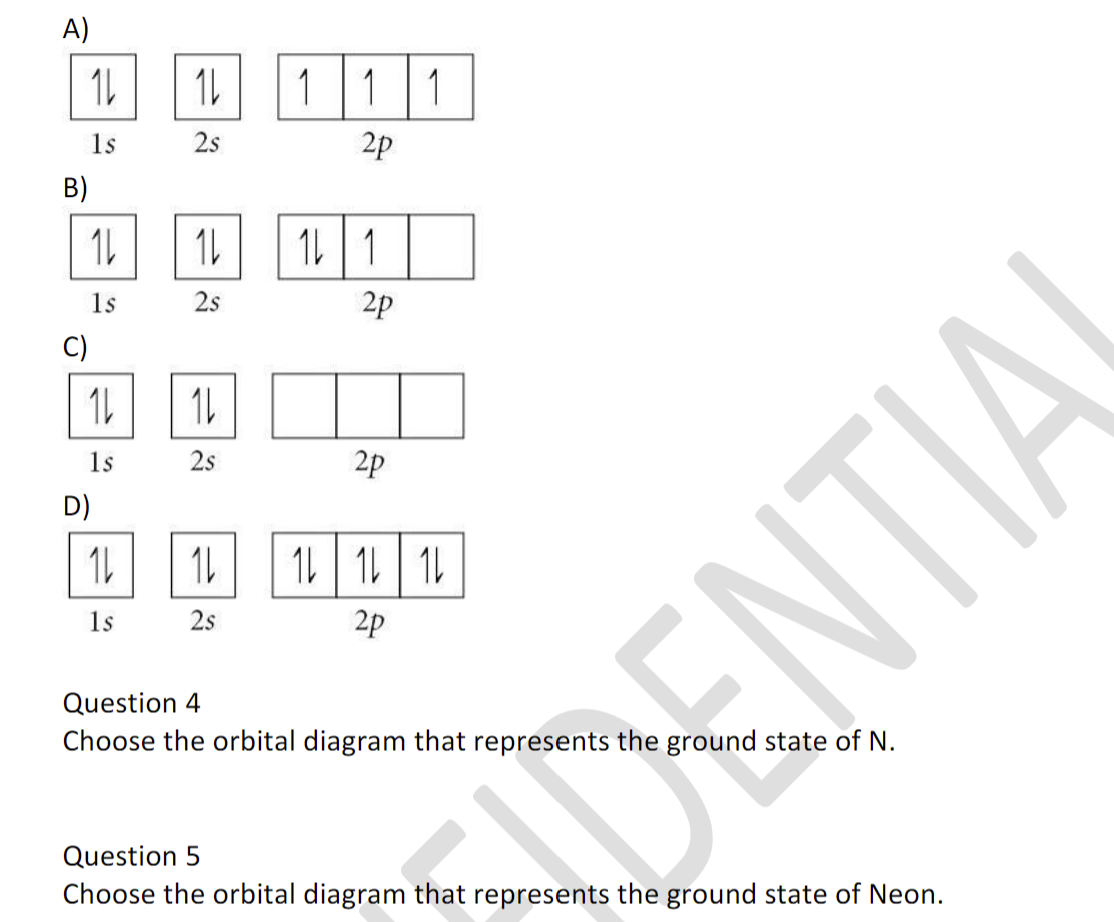
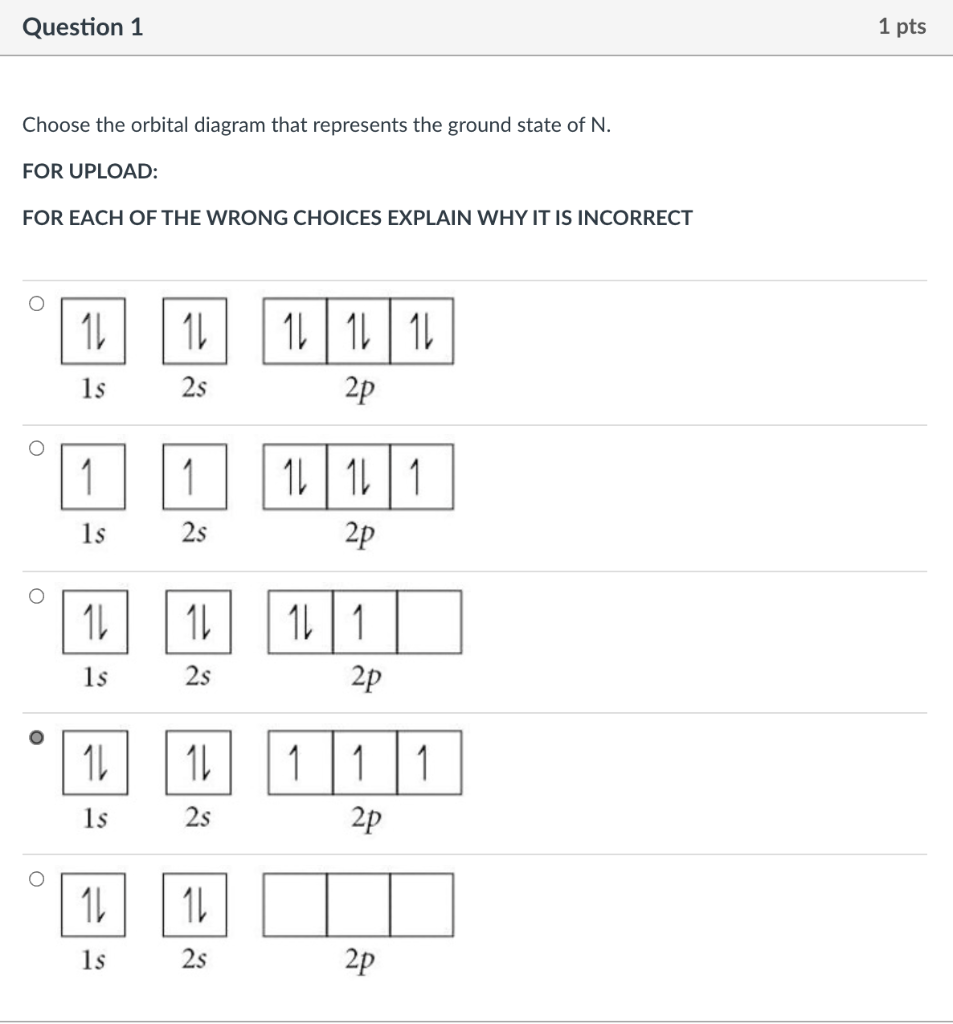
39 choose the orbital diagram that represents the ground state of n.
View Chapters 9-11 Worksheet_Final Exam.docx from CHEM 125 at New Jersey Institute Of Technology. Chapters 9-11 Work sheet Questions 1) Choose the orbital diagram that represents the ground state of
Choose the orbital diagram that represents the ground state of N. Choose the valence orbital diagram that represents the ground state of Zn. Give the ground state electron configuration for Se. [Ar]4s23d104p4. Give the ground state electron configuration for I. [Kr]5s24d105p5.
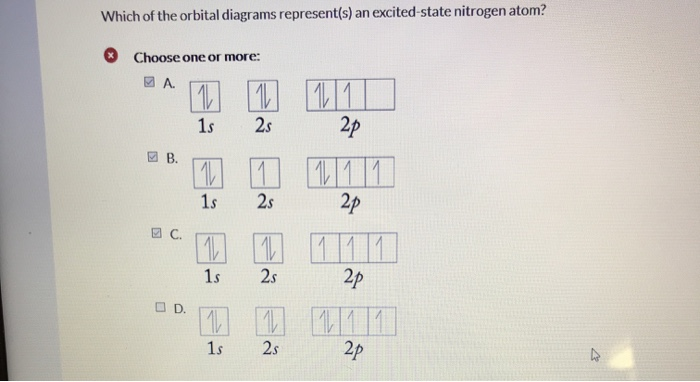
Choose the orbital diagram that represents the ground state of N. ... Which of these electron diagrams could represent the ground state of the p valence electrons of carbon? Of the following listed orbital choices - 1p, 2d, 3d, & 4f , only _____ can exist.a. 1pb. 3dc. 2d and 3dd. 3d, and 4fe. 4f ...
Choose the orbital diagram that represents the ground state of n.
Choose the valence orbital diagram that represents the ground state of Ni. asked Jul 31, 2019 in Chemistry by kiwis. general-chemistry.
How to Write the Electron Configuration for Nitrogen (N) Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital.
D) E) Answer: E 15) Write out the orbital diagram that represents the ground state of As. How many unpaired electrons are there? A) 0 B) 4 C) 3 D) 2 E) 1 Answer : C
Choose the orbital diagram that represents the ground state of n..
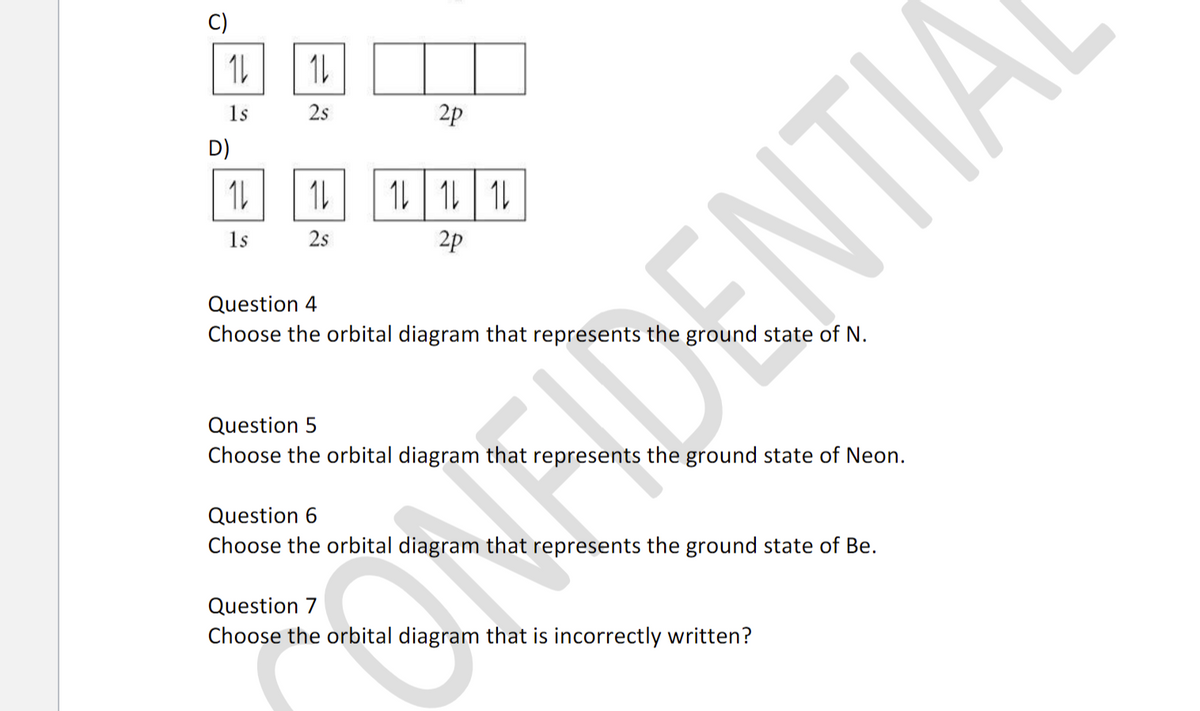
Question 4-7 refers to the following orbital diagram A) 1 1 1 1s 2s 2p B) 1L 1L 1 1s 2s 2p C) 1s D) 2s 2p 1L | 11 | 11 1s 2s 2p Question 4 Choose the orbital diagram that represents the ground state of N. Question 5 Choose the orbital diagram that represents the ground state of Neon.
12) Give the set of four quantum numbers that represent the last electron added (using the Aufbau principle) to the Cl atom. A) n = 3, l =1 , ml = 0, ms = -
Ground or Excited State for Nitrogen. In question 1.69 (b), there is a picture which shows the electron configuration for Nitrogen. There are two arrows for the 1s orbital, 2 arrows in the 2s orbital, and one arrow in each of the three 2p orbitals. The question asks us to determine whether the electron configuration represents the excited state ...
Page 2 A)Li B)Si C)Al D)Cl 13.The diagram below represents the orbital notation of an atom's valence shell in the ground state. The diagram could represent the valence shell of
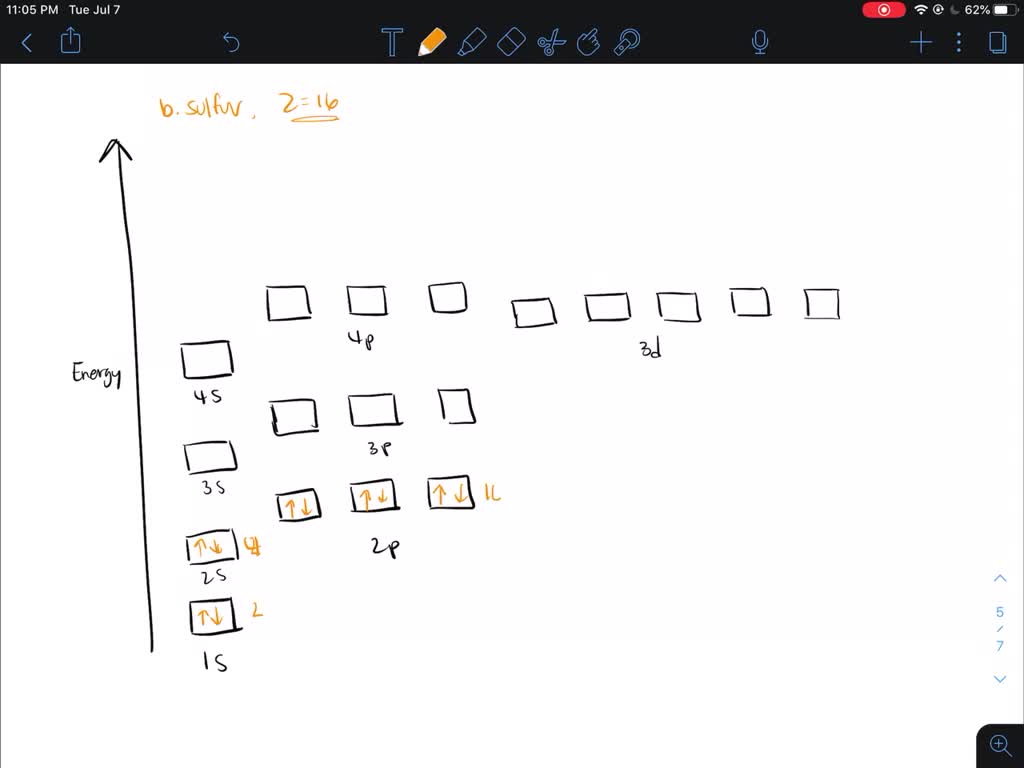
Choose the orbital diagram that represents the ground state of n. Give the ground state electron configuration for se. Choose the orbital diagram that represents the ground state of n. Identity the element that has a ground state electronic configuration of kr5s24d5. Choose the ground state electron configuration for ti2.
Choose the orbital diagram that represents the ground state of N. 1 The condensed electron configuration of silicon, element 14, is_ [He]2s42p6 b. [Ne]2p10 [Ne]3s23p2 d. [He]2s4 [He]2s62p2 Enthalpy is an expression for the heat content b. energy state c. reaction rate d. activation energy If AH reaction is -100 kcal/mol, it indicates the ...
Question 2 05 out of 05 points choose the statement that is true. Choose the orbital diagram that represents the ground state of n. Orbital diagram that represents the ground state of n 1s2 2s2 2p3 give the set of four quantum numbers that could represent the last electron added using the aufbau principle to the cl atom.
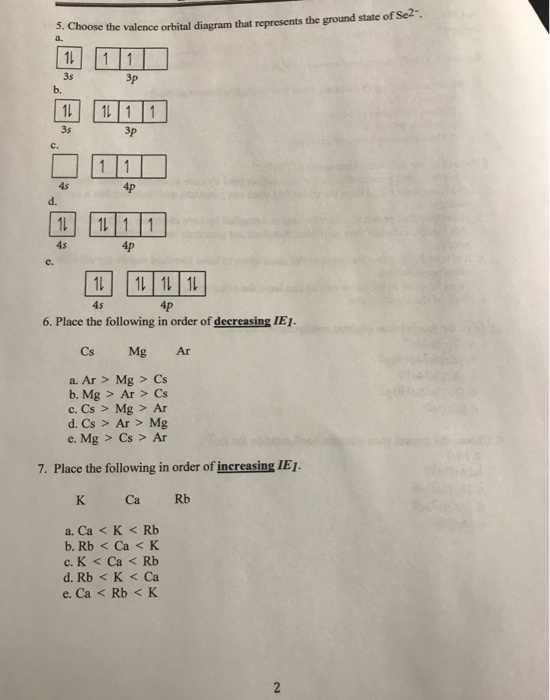
Choose the orbital diagram that represents the ground state of N. Give the ground state electron configuration for Se. [Ar]4s23d 104p4 [Ar]4s24dl04p4 [Ar]4s23dl04p6 [Ar]4s23dl0 [Ar]3dl04p4 Identify the clement that has a ground state electronic configuration of [Kr)5s24d5.
Choose the valence orbital diagram that represents the ground state of zn. 11 years aieee chapterwise by mtg hope it helps by shreesha rao 2 in types school work the d block elements lardbucket alloys and pounds of the d block elements are important ponents of the materials the modern world depends on for its continuing technological. 5 choose ...
The above diagram roughly depicts the relative energy difference between these three ways of filling 2 electrons into the three p orbitals. Ground State: 1s22s22p x 12p y 1 (or 1s22s22p x 12p z 1 or 1s22s22p y 12p z 1) [CAUTION: these don't explicitly state the electron's spin!]
1s 2s 2p D) 1L | 11 1 1s 2s 2p Question 4 Choose the orbital diagram that represents the ground state of N. uDENTIA Question 5 Choose the orbital diagram that represents the ground state of Neon. Question 6 Choose the orbital diagram that represents the ground state of Be. Question 7 Choose the orbital diagram that is incorrectly written?
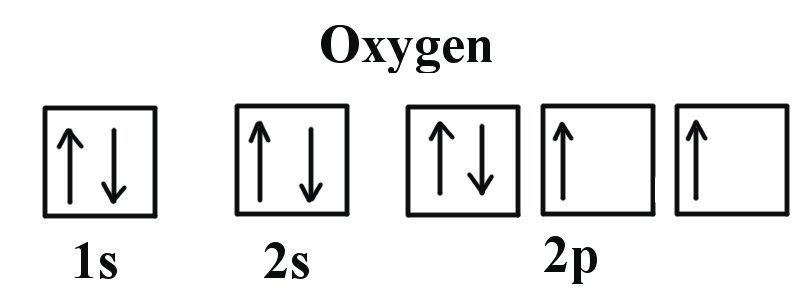
Based on the periodic table, "O" is atomic number 8, which means it has 8 electrons. The first few atomic orbitals are 1s, 2s, and 2p. Each orbital can hold 2 electrons maximum, and there are 2l+1 of each type of orbital (s,p,d,f,g,...), where l = 0 corresponds to an s orbital, l = 1 means p orbital, and so on. So, the configuration for neutral "O" atom is: 1s^2 2s^2 2p^4 where the 2p orbitals ...
Orbital diagram that represents the ground state of n 1s2 2s2 2p3 give the set of four quantum numbers that could represent the last electron added using the aufbau principle to the cl atom. Be sure to. 5 choose the valence orbital diagram that represents the ground state of zn.
Choose the orbital diagram that represents the ground state of N. Learn this topic by watching The Electron Configuration Concept Videos All Chemistry Practice Problems The Electron Configuration Practice Problems
Ground State Electron Configuration For Nitrogen. When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s 2 2s 2 2p 3.Below you can get the full image representation which will help you to understand the topic more easily.
Choose the orbital diagram that represents the ground state of n. The orbital diagram for a ground state nitrogen atom is. The electron configuration of a ground state co atom is 1b a ar4s24d7 b ar4s13d5 c 1s22s22p63s23d9 d ar4s23d7 e ne3s23d7.
Determine the end (final) value of n in a hydrogen atom transition, if the electron starts in n = 1 and the atom absorbs a photon of light with an energy of 2.044 * 10^-18 J 3; 4; 2; 5; 6; How many different values of l are possible in the third principal level? 1; 2; 3; 0; 4; Give the ground state electron configuration for Pb [Xe]6s2 6p2 [Xe ...
Choose the orbital diagram that represents the ground state of N. 1s:^> 2s:^> 2p:^^^; >=down arrow; Nitrogen has atomic # of 7. Choose the valence orbital diagram that represents the ground state of Zn. 4s:^>(4s^2) 3d:^> ^> ^> ^> ^> (3d^10) The element that corresponds to the electron configuration 1s^2 2s^2 2p^6 3s^2 3p^6 4s^1 3d^5 is _____. iron
What orbital diagram correctly represents the outermost principal energy level of a nitrogen atom in the ground state? 1S22S23P3 1S22S23P3 What atom is represented in the following orbital diagram ...
Here's what I got. Your starting point here will be tin's electron configuration. Tin, "Sn", is located in period 5, group 14 of the periodic table and has an atomic number equal to 50. This tells you that a neutral tin atom will have a total of 50 electrons surrounding its nucleus. So, the electron configuration for tin looks like this - I'll use the noble gas shorthand notation "Sn: " ["Kr ...
Ans- (b) As Nitrogen contains 7 electrons in its ground state. 1st two electrons w …. View the full answer. Transcribed image text: Choose the orbital diagram that represents the ground state of N. Previous question Next question.
CHE 201-121 Exam 5 Ch 7 Atomic Structure (7.5-7.13) Sample Questions Summer, 2017 Choose the orbital diagram that represents the ground state of N. 20. Choose the valence orbital diagram that represents the ground state of Zn. 28.















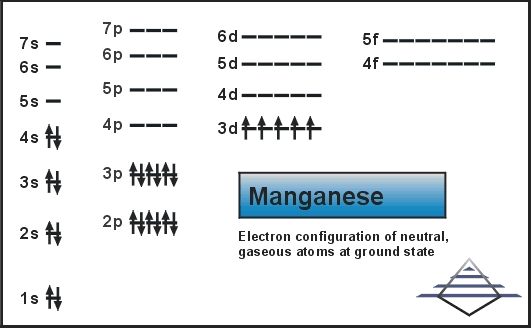










0 Response to "39 choose the orbital diagram that represents the ground state of n."
Post a Comment