39 sn2 reaction coordinate diagram
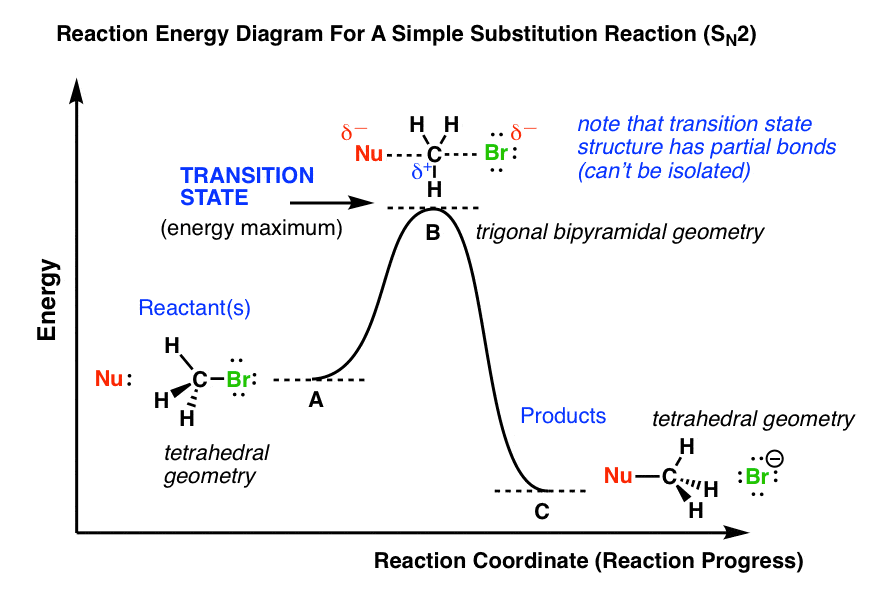
The SN2 reaction mechanism stands for substitution nucleophilic bimolecular and is a fundamental mechanism in organic chemistry. In an SN2 mechanism, a nucle... SN2 reaction coordinate diagram. In this diagram, there are really only three parts: the reagents, the transition state, and the products. The transition state is the point in the reaction with the highest energy level, and the difference in energy between the reagents and transition state is called the activation energy (often abbreviated as Ea). This Pin was discovered by Jessica L. Santos ...
SN2 reaction Not effected but low concentration disfavors a SN2 reaction Protic polar favors a SN1 reaction if the reactant is not charged. Ex: H2O, CH3OH, etc. Racemization (with some inversion because of ion pairing) E1 3>2>1 Forms a carbocation Weak base favors E1 reaction by disfavoring E2 reaction Not effected but a low concentration of base

Sn2 reaction coordinate diagram
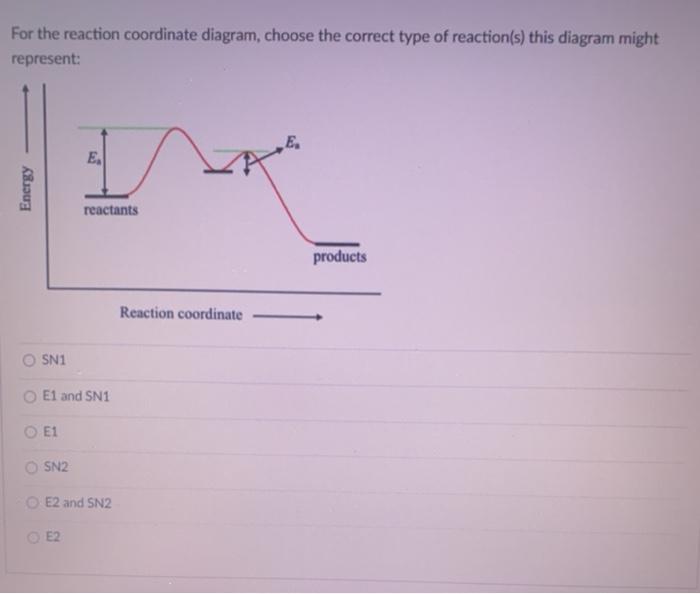
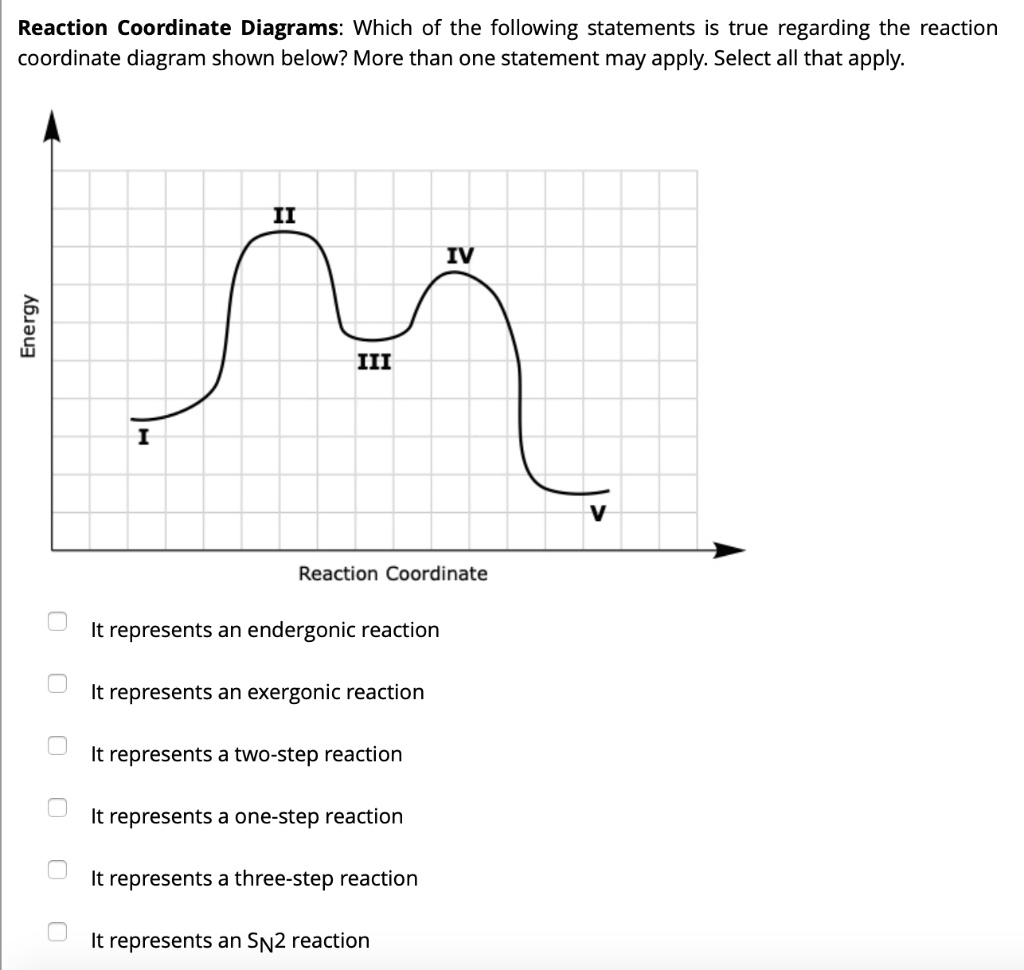
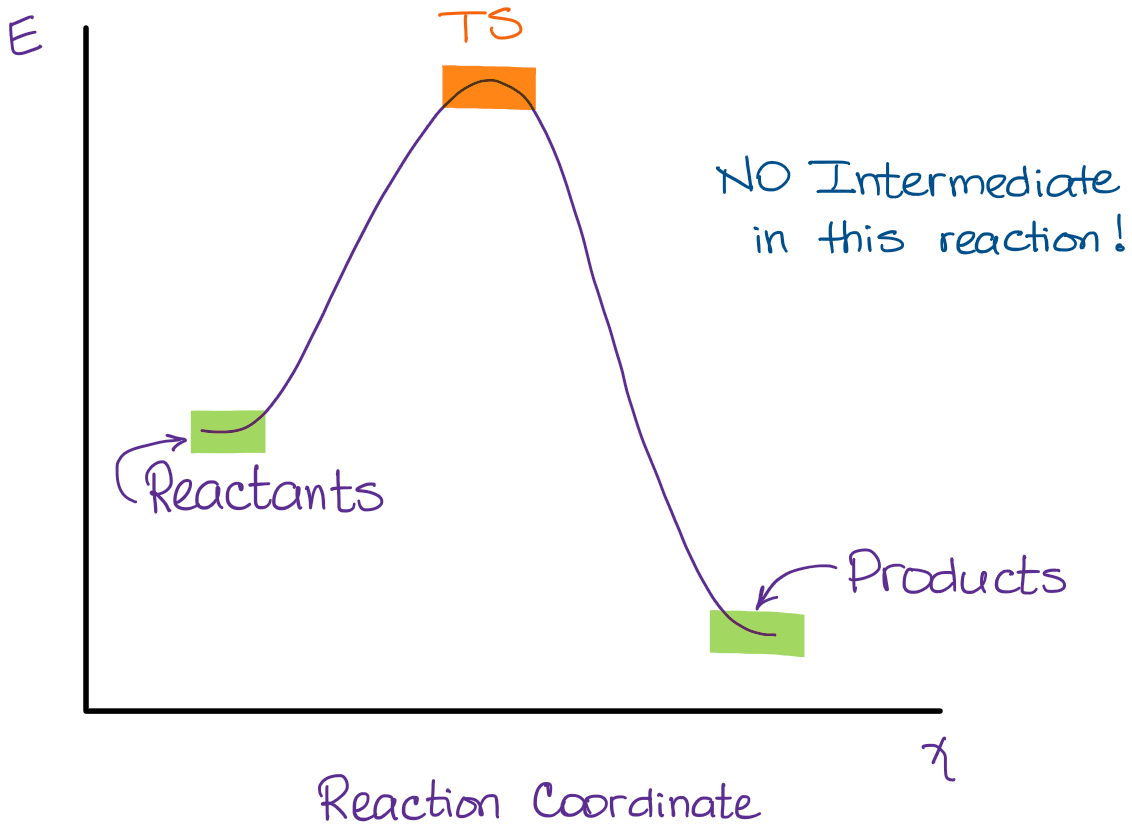
Select all the statements that correctly describe the reaction coordinate vs. energy diagram for an SN1 reaction. A. The energy diagram shows two energy maxima corresponding to the 2 transition states B. The carbocation intermediate is more stable than the starting material. C. SN2 REACTIONS Introduction ... Transition state represents an energy maximum on the reaction coordinate and can’t be measured directly due to their extremely short lifetime interval. Their lifetime is approximately 10-12 seconds. In bimolecular reactions, the transition state represents one specific orientation of the reactants. The entropy of activation, ΔS is negative. In the beginning of ... (simultaneously); there are no intermediates. Although the reaction coordinate energy diagram illustrates the activation energy and the ΔG°, we are often interested only in the shape of the diagram: for the SN2 reaction, that there is one transition state, one step. For now, the quantities for E a and ΔG° are not important.
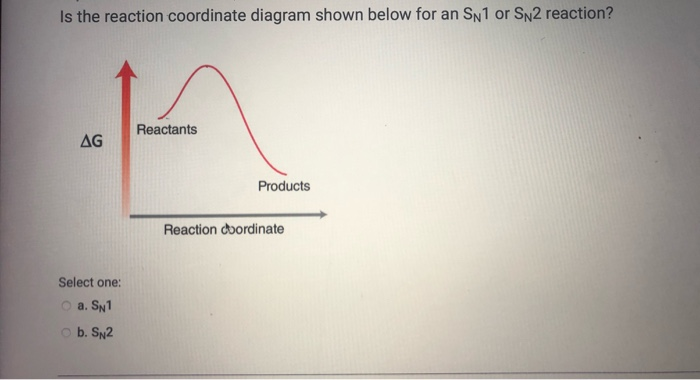
Sn2 reaction coordinate diagram. S N 2 mechanism. S N 2 indicates a substitution, nucleophilic, bimolecular reaction, described by the expression rate = k [Nu][R-LG]. This implies that the rate determining step involves an interaction between two species, the nucleophile and the organic substrate. This pathway is a concerted process (single step) as shown by the following reaction coordinate diagrams, where there is ... Unformatted text preview: ! 1 CHEM 232 Worksheet SN2 1. Draw the reaction coordinate diagram for the SN2 reaction of 1-iodopropane with -NH2 to form a neutral organic product. Be sure to include the starting materials, any intermediates, and products on the diagram. Draw the transition state for the rate-determining step. SN1 is a two-stage system, while SN2 is a one-stage process. The carbocation can form as an intermediate during SN1 reactions, while it is not formed during SN2 reactions. 3. What determines sn1 or sn2? Ans: In the rate of reaction, Sn1 reactions are unimolecular and have a step-wise mechanism. Figure 2 identifies these species in a reaction coordinate diagram like the one in the right-hand panel of Figure 1. Here the nucleophile is hydroxide ion. Figure 2 The 2-Step. As the figure indicates, the activation energy for the first step of the reaction is much larger than that for the second step. Thus the first step is much slower.
Transition state and energy diagram of an S N 2 reaction: Chloroform hydrolysis. Due to their extremely short lifetime, transition states cannot be measured directly. They represent an energy maximum on the reaction coordinate. Their lifetime does not last any longer than one molecular vibration, which is approximately 10-12 s. In bimolecular reactions, such as in an S N 2 reaction, the ... SN2 mechanism. SN2 indicates a substitution, nucleophilic, bimolecular reaction, described by the expression rate = k [Nu][R-LG] . This pathway is a concerted process (single step) as shown by the following reaction coordinate diagrams, where there is simultaneous attack of the nucleophile and displacement of the leaving group. Construct the gas phase Reaction Coordinate Diagram for the Cl + CH3Cl SN2 reaction by plotting the relative energy in kJmol 1 versus Cl + C | {z} Distance H3 Cl. Place the Reaction Coordinate Diagram on the graph provided below and plot the energies on a relative energy scale. Label the various species along the reaction pathway. 0 5 10 15 20 25 30 35 40 45 50 55 4.0 3.5 3.0 2.5 2.0 1.8 2.0 2 ... http://Leah4sci.com/substitution-elimination presents: SN2 Energy Diagram Need help with Orgo? Download my free guide '10 Secrets to Acing Organic Chemistry'...
Figure 1: SN2 reaction showing concerted, bimolecular participation of nucleophile and leaving group. A consequence of the concerted, bimolecular nature of the S N 2 reaction is that the nucleophile must attack from the side of the molecule opposite to the leaving group. This geometry of reaction is called back side attack. In a back side ... This pathway is a concerted process (single step) as shown by the following reaction coordinate diagrams, where there is simultaneous attack of the nucleophile and ... nucleophile is very important in an SN2 reaction. The more reactive the nucleophile, the more likely the reaction will be SN2 rather than SN1. SN2 reaction coordinate diagram. In this diagram, there are really only three parts: the reagents, the transition state, and the products. The transition state is the point in the reaction with the highest energy level, and the difference in energy between the reagents and transition state is called the activation energy (often abbreviated as Ea). The S N 1 reaction energy diagram illustrates ... Substitution Reactions (SN2 versus SN1). Substrate: Sterics reaction coordinate (SN1) en er g y en erg Generic Reaction-Energy Diagrams. Predicting the. SN1 indicates a substitution, nucleophilic, unimolecular reaction, described by the expression rate = k reaction coordinate diagram for a two step process. SN1 reaction The S1 reaction is a ...
Potential Energy Diagram for S N 2! Reaction Coordinate! CH3O H3CCl H HH H3CO Cl CH3OCH3 Cl Bond is forming! Bond is breaking! H HH Transition state in a S N 2 reaction resembles a sp2 hybridized carbon! NUC! LG! Species in a Given S N 2 Reaction! nucleophile! electrophile! transition state! products!
The mechanism, rate law, and stereochemistry of Sn2 reactions. How the sterics of the alkyl halide affect the reaction rate. Created by Jay. Sn1 and Sn2. Identifying nucleophilic and electrophilic centers. Curly arrow conventions in organic chemistry. Intro to organic mechanisms. Alkyl halide nomenclature and classification.
reaction coordinate Br Figure 9.11 Reaction free-energy diagram for the S N1-E1 solvolysis reaction of (CH 3) 3CBr with ethanol.The rate-limiting step,ionization of the alkyl halide (red curve),has the transition state of highest standard free energy.The
SN2 reaction coordinate diagram. In this diagram, there are really only three parts: the reagents, the transition state, and the products. The transition state is the point in the reaction with the highest energy level, and the difference in energy between the reagents and transition state is called the activation energy (often abbreviated as Ea). Remember that the rate-determining step is the ...
A diagram of the reaction of the SN2 type substitution coordinate between methyltosylate and nucleophile iodide and chloride are presented in the graph below. Based on the relative potential energy, the CH3-Cl bond is stronger than the CH3-I bond, so the SN2 product (methylchloride) has a lower energy than methyl iodide. Based on the results of.
S N 2 mechanism. S N 2 indicates a substitution, nucleophilic, bimolecular reaction, described by the kinetic expression : rate = k [Nu][R-LG] . This pathway is a concerted mechanism (single step) as shown by the following reaction coordinate diagrams, where there is simultaneous attack of the nucleophile and displacement of the leaving group.
Sn1 Reaction Coordinate Diagram. SN1 reaction is a two step reaction as mentioned below: 1. Leaving group leaves first being solvolysed by solvent creating a carbocation intermediate. This is. whose proposed mechanism and free energy diagram are depicted Figures 1 and 2. Figure 2: Reaction coordinate diagram for an SN1 reaction1. 1.
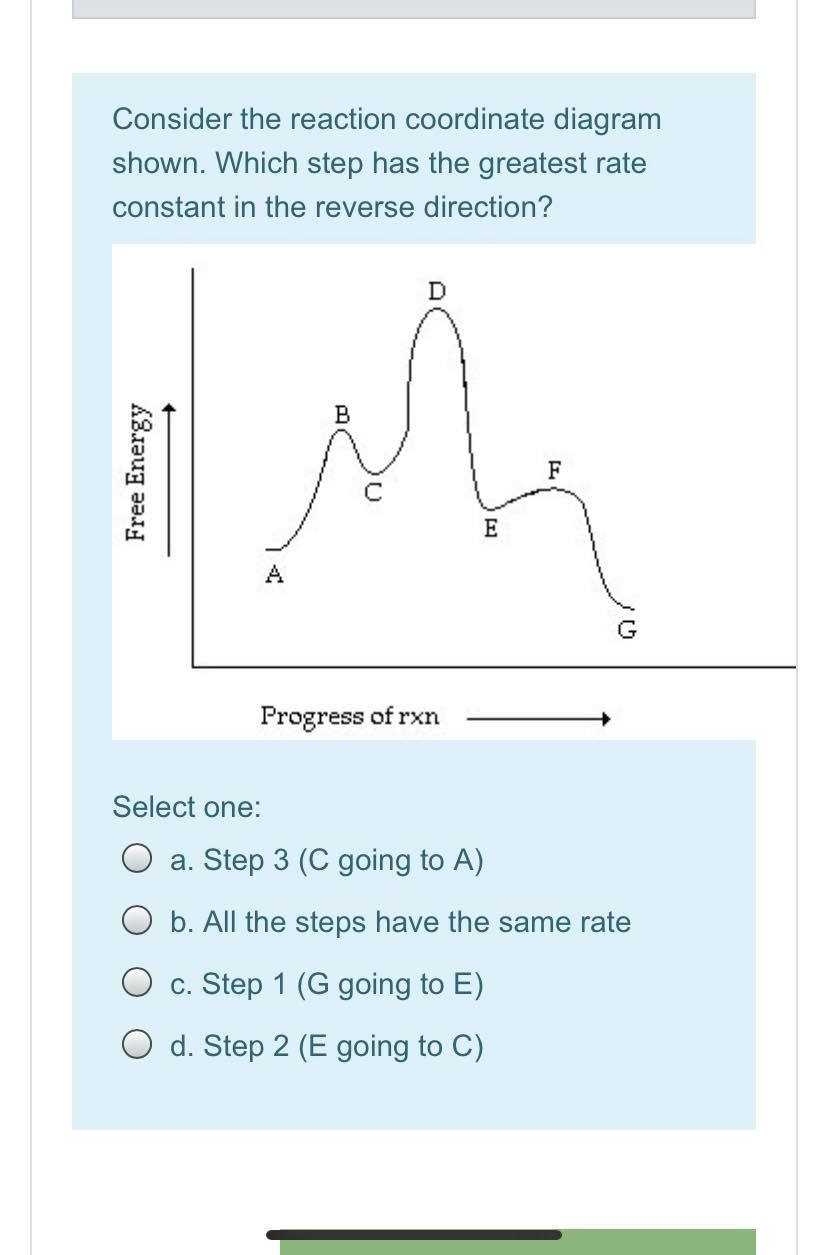
The three general rules for reaction coordinate diagrams are as follows: ... Does Wurtz-Fittig-reaction involves Sn1 or Sn2? 2. How to explain the selectivity between methanol and methanethiol in an SN1 reaction with an halogenated hydrocarbon? 0. 2-step reaction mechanism for the decomposition of NO2Br. 2.
The S N 2 Reaction Notes: In the SN2 reaction, the nucleophile attacks from the most δ+ region: behind the leaving group. This is called a back-side attack. This back-side attack causes an inversion (study the previous slide): after the leaving group leaves, the other substituents shift to make room for the newly-bonded nucleophile, changing the stereochemistry of the molecule.
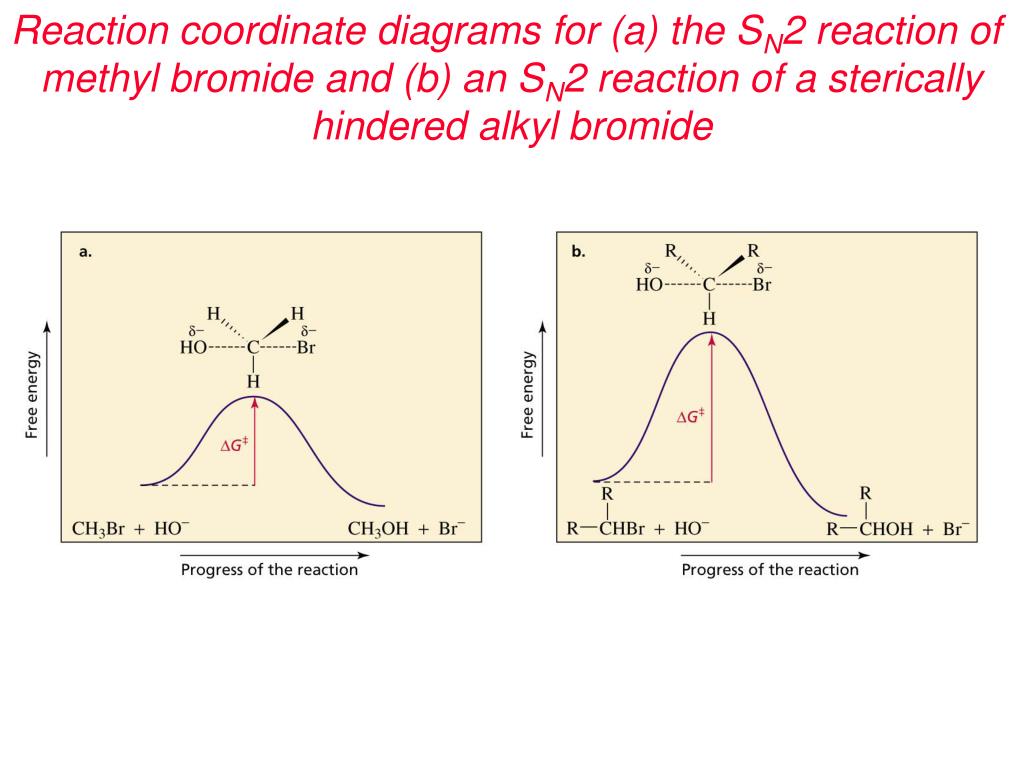
Figure 9.3 shows the reaction coordinate diagram in the S N 2 reaction of hydroxide ion with chloromethane to give methanol and chloride ion. We see that the transition state contains both hydroxide ion and the substrate. As the reaction proceeds through the transition state, a bond forms between carbon and hydroxide ion, and the bond between carbon and chlorine breaks.
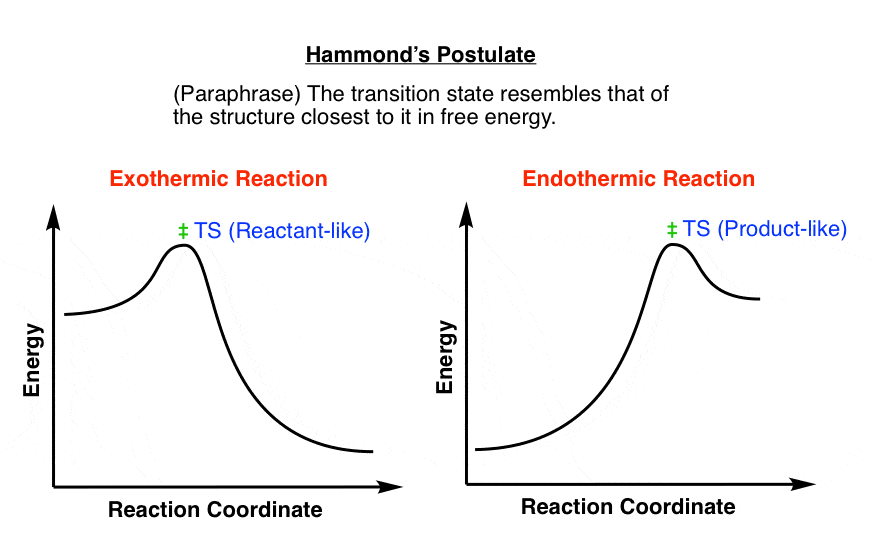
You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the 'reaction coordinate', tracing from left to right the progress of the reaction from starting compounds to final products.
(simultaneously); there are no intermediates. Although the reaction coordinate energy diagram illustrates the activation energy and the ΔG°, we are often interested only in the shape of the diagram: for the SN2 reaction, that there is one transition state, one step. For now, the quantities for E a and ΔG° are not important.
SN2 REACTIONS Introduction ... Transition state represents an energy maximum on the reaction coordinate and can’t be measured directly due to their extremely short lifetime interval. Their lifetime is approximately 10-12 seconds. In bimolecular reactions, the transition state represents one specific orientation of the reactants. The entropy of activation, ΔS is negative. In the beginning of ...
Select all the statements that correctly describe the reaction coordinate vs. energy diagram for an SN1 reaction. A. The energy diagram shows two energy maxima corresponding to the 2 transition states B. The carbocation intermediate is more stable than the starting material. C.










/chapter7/pages5and6/page5and6_files/sn2phenoxyenergy.png)

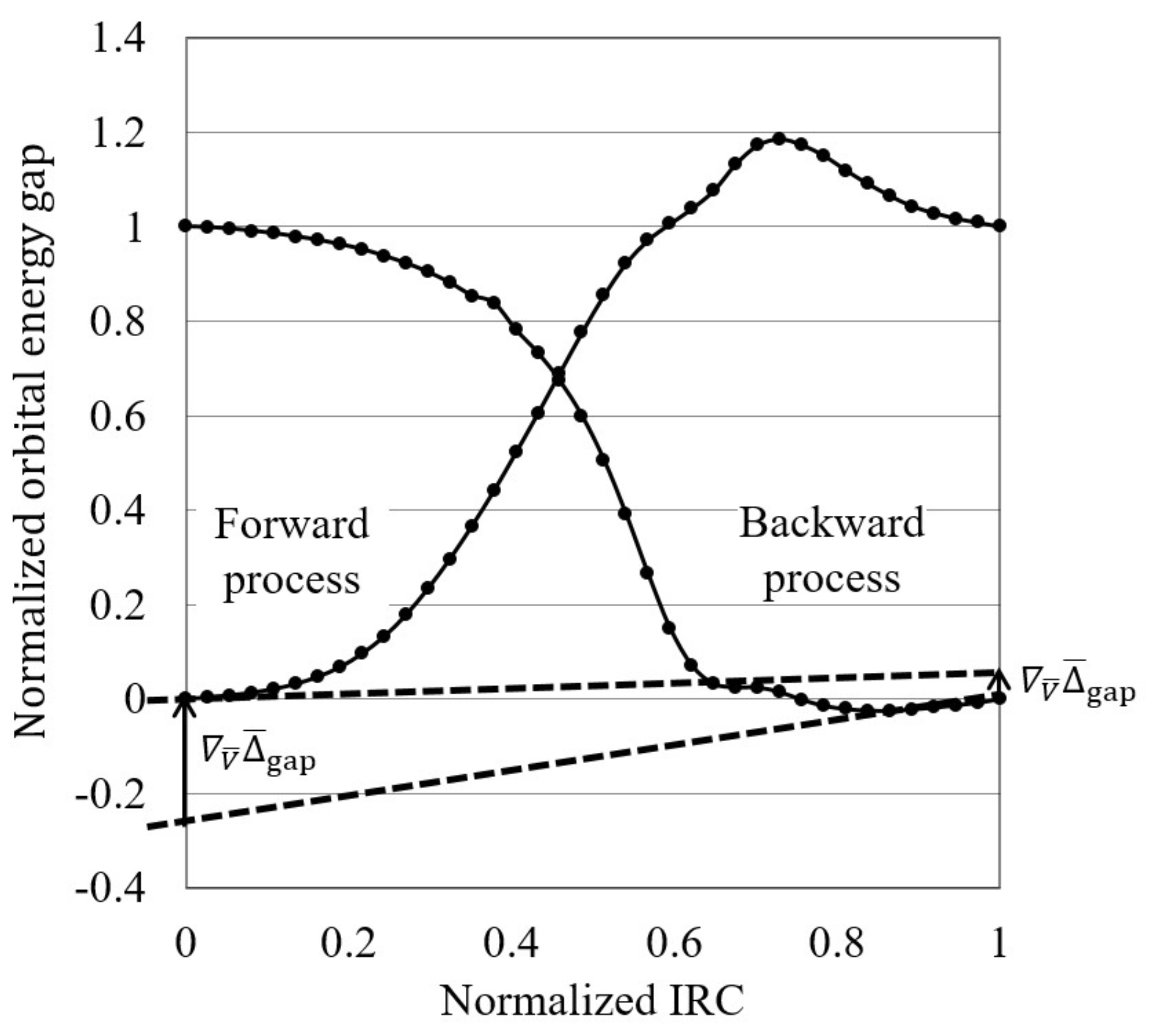
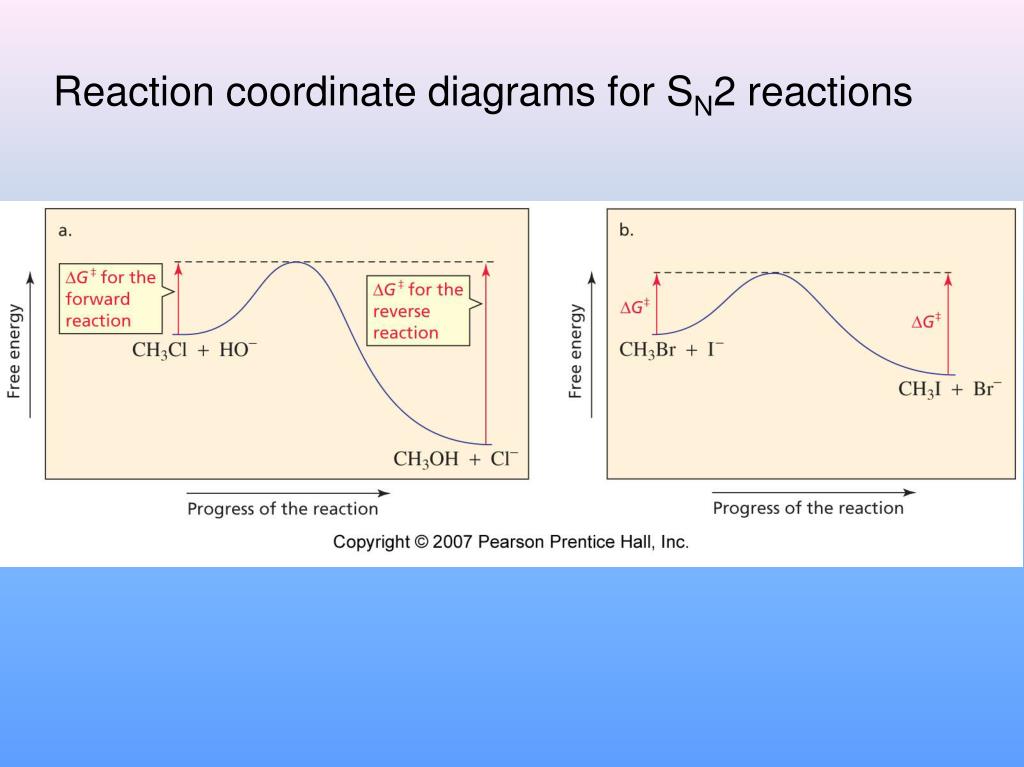

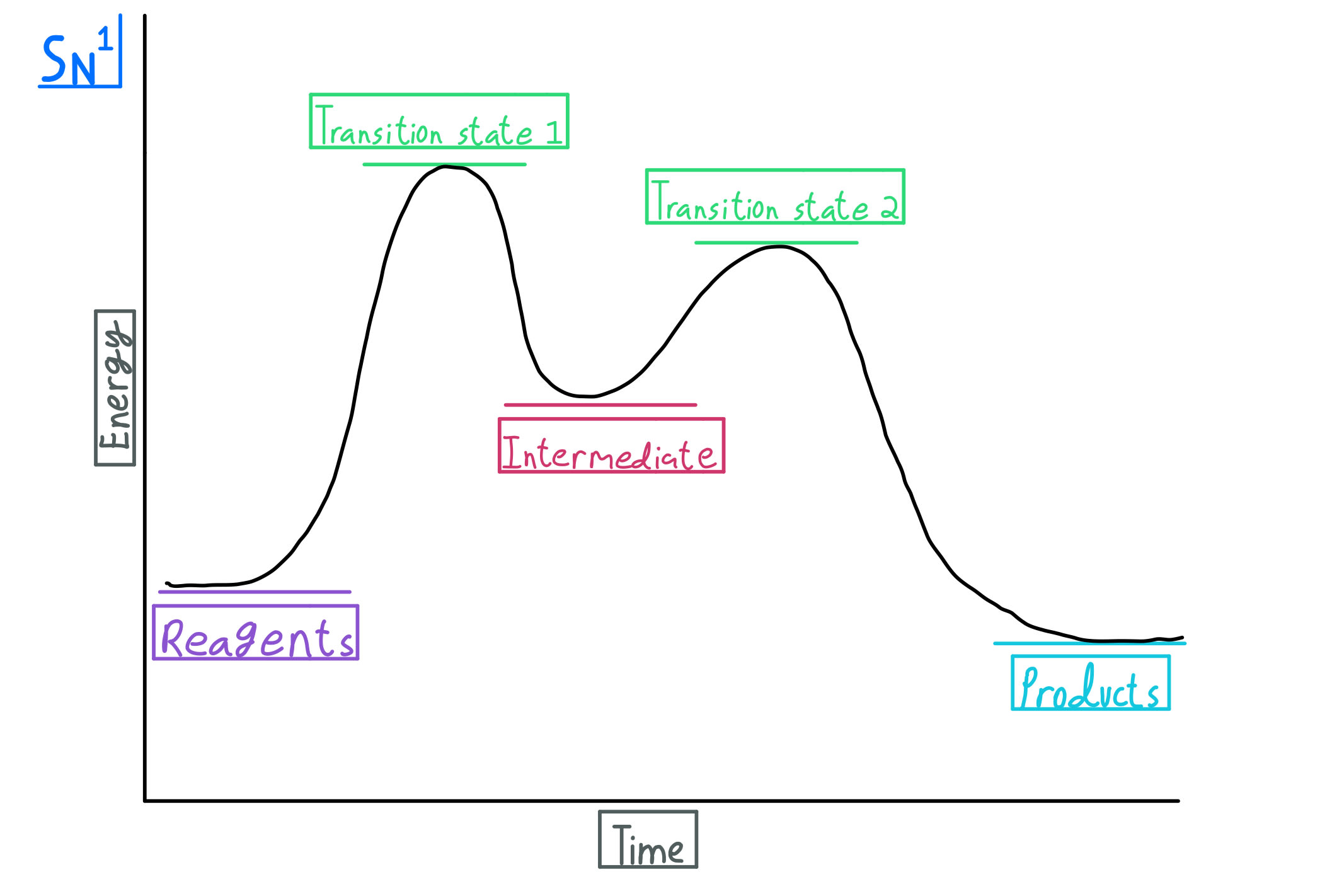
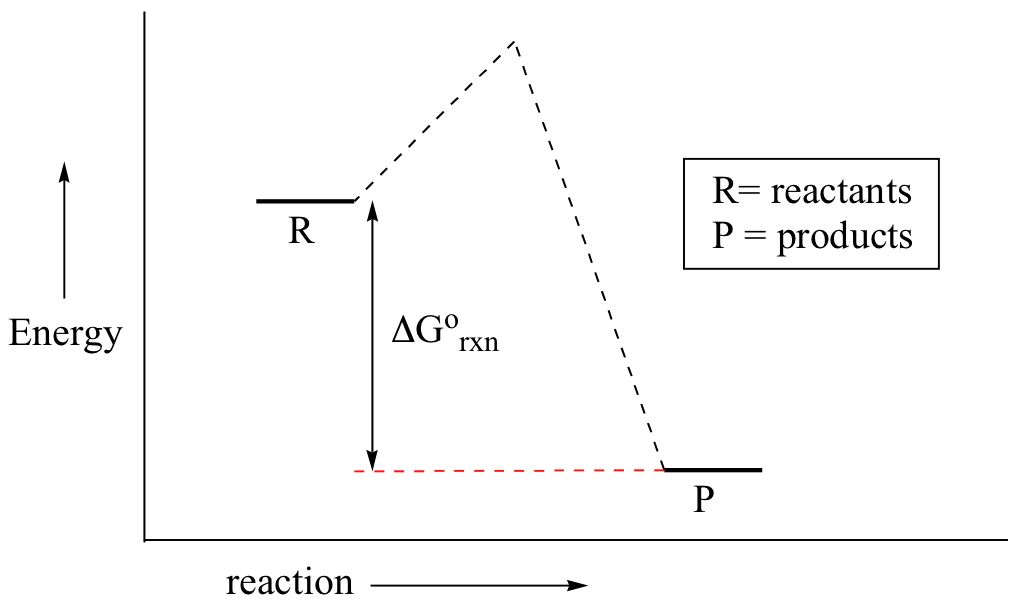







0 Response to "39 sn2 reaction coordinate diagram"
Post a Comment