39 Bn Molecular Orbital Diagram
Molecular Orbital Theory - ppt video online download Presentation on theme: "Molecular Orbital Theory"— Presentation transcript: 1 Molecular Orbital Theory Edward A. Mottel Department of Chemistry Rose-Hulman Institute of Technology. 2 Bonding Theories Ionic Model Skeleton Diagrams Lewis Dot Diagrams Formal Charge... Molecular orbital diagrams - Overleaf, Online LaTeX Editor Molecular orbital diagrams provide qualitative information about the structure and stability of the electrons in a molecule. This article explains how to create molecular orbital diagrams in LaTeX by means of the package MOdiagram. For information about the more traditional molecular structure...
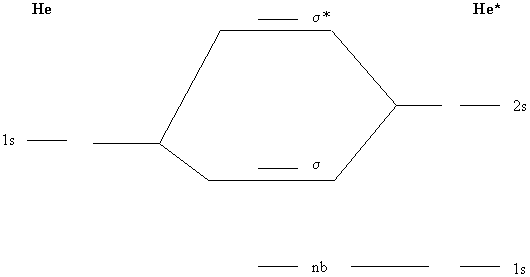
Molecular Orbital Theory This molecular orbital model can be used to explain why He2 molecules don't exist. Combining a pair of helium atoms with 1s2 electron configurations would produce a molecule with a pair of electrons in both the bonding and the * antibonding molecular orbitals. The total energy of an He2 molecule...
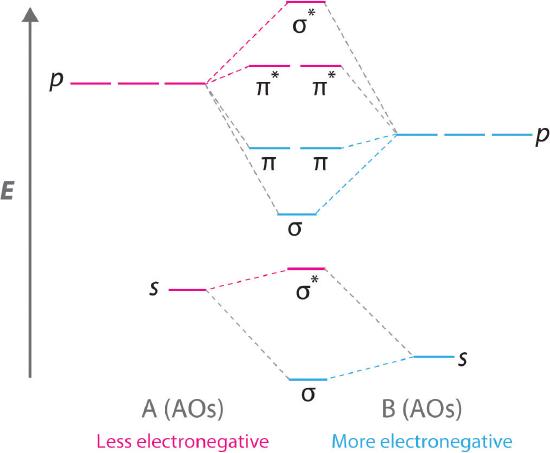
Bn molecular orbital diagram
Molecular Orbital Diagrams - Every Science The procedure for working out a molecular orbital of a general diatomic molecule is quite simple. We construct molecular orbitals using the available orbitals on the atoms. We then fill up the molecular orbitals, starting with the lowest in energy, until all the electrons in the species have been assigned to... Molecular Orbital Diagram Of Bn | Molecular, Molecular shapes... Sign in | Etsy. Laminated hand-drawn human anatomy study diagrams. Life Science & Biology with Mel and Gerdy. Day 06 3 Molecular Orbital (MO) Theory for BN - YouTube Professor Chang Y. RyuDepartment of Chemistry and Chemical Biology, RPIGeneral Chemistry Discussion Problems.
Bn molecular orbital diagram. The Pi Molecular Orbitals of Butadiene And How To Draw Them The Lowest-Energy Molecular Orbital (π1) Of The Butadiene Pi System Has Zero Nodes The Full Molecular Orbital Diagram For The Butadienyl System (n=4) A molecular orbital diagram without electrons is like an apartment building without people. PDF Microsoft PowerPoint - Polyatomic Molecular Orbital Theory... Polyatomic Molecular Orbital Theory. Transformational properties of atomic orbitals. • When bonds are formed, atomic orbitals combine according to their symmetry. The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row What is the molecular orbital energy diagram of CO? - Quora A molecular orbital diagram , or MO diagram , is a qualitative descriptive tool explaining chemical bonding. The electronic of hydrogen and fluorine are 1s¹ and 1s²2s²2p⁵ respectively. In the formation of HF molecule ,only 2p electrons of fluorine atom would combine effectively with the solitary electron...
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... • Molecule orbital theory (Robert Mullikan). • Electrons are delocalised - Different to Lewis and hybridisation - Molecular orbitals are formed which involve all of the atoms of the molecule. • Energy level diagram represents this interaction. - Two s orbitals interaction to create a low energy... An introduction to molecular orbital theory The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative calculations, including those that lead to many of the computer-generated images that you have seen elsewhere in these units. Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons. Molecular Orbital Theory - GeeksforGeeks The Molecular Orbital Theory is a chemical bonding theory developed at the turn of the twentieth century by F. R. Hund and R. S. Mulliken to explain the structure and properties of various molecules. The valence-bond theory failed to adequately explain how certain molecules, such as...
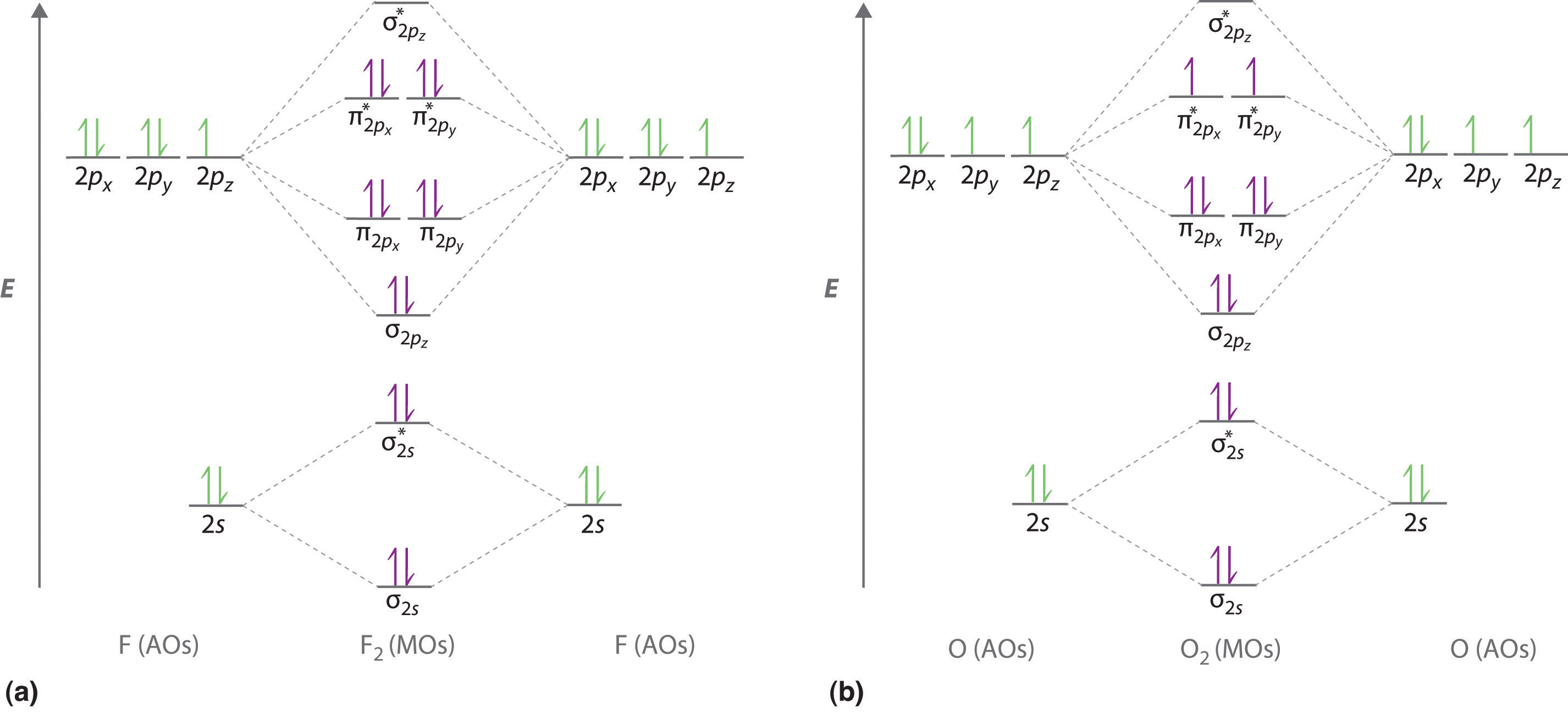
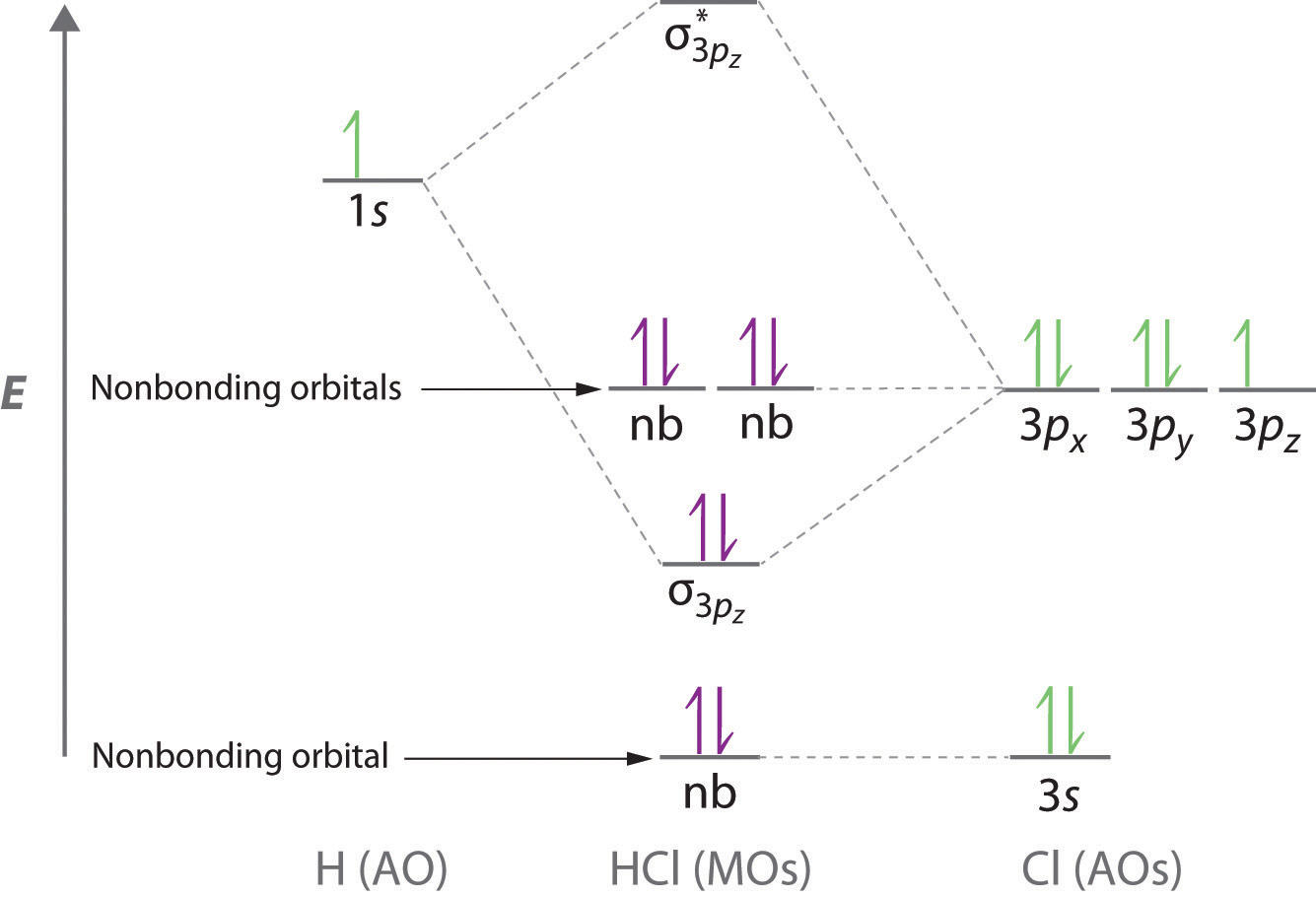
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. 8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. Molecular Orbital Theory: Explanation, Illustrations and... - Embibe Molecular Orbital Theory: To simplify things, we will consider the interaction of the orbitals containing valence electrons to create molecular orbitals. Have you ever thought about how sigma and pi bonds are formed? What is the difference between diamagnetic and paramagnetic behaviour? Molecular Orbital Theory (MOT), Chemistry Study... | eMedicalPrep The molecular orbital diagram representing this order of energy levels is shown in fig. No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if the value is negative or zero, it means that the molecule is unstable.
Molecular Orbital Diagrams simplified | by Megan A. Lim | Medium Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory. Valence Bond Theory proposes that electrons are localized between two atoms.
Molecular Orbital Theory Molecular Orbital Theory Bonding molecular orbital: describes regions of high electron probability or charge density in the internuclear region between two Molecular Orbital Theory Problem: Write a molecular orbital diagram and determine the bond order for: (a) Ne2+ (b) C22Problem: Using...
8.4 Molecular Orbital Theory - Chemistry Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described...
Asked for: molecular orbital energy-level diagram, valence electron... A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Just as with atomic orbitals, we create an energy-level diagram by listing the molecular orbitals in order of increasing energy. We then fill the orbitals with the required number...
Molecular Orbital Theory Diagram The molecular orbital theory is one of the most productive models of chemical bonding. It is the basis of quantitative calculations, including those regarding the The molecule consists of two nuclei having charge +1 and shares a single electron between them. When two hydrogen nuclei move towards each...
Molecular Orbital Theory | Chemistry [Master] Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the...
PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams In molecular orbital theory, we postulate that. the combination of atomic orbitals on different atoms forms molecular orbitals (MOs), so that electrons Diagrams such as these are used to describe the bonding in a molecule in MO terms. Electrons occupy MOs according to the same rules developed...
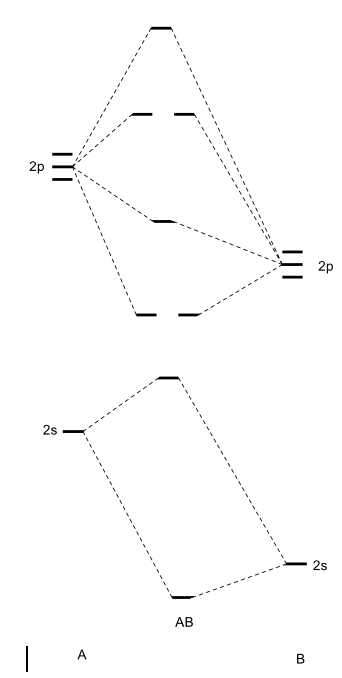
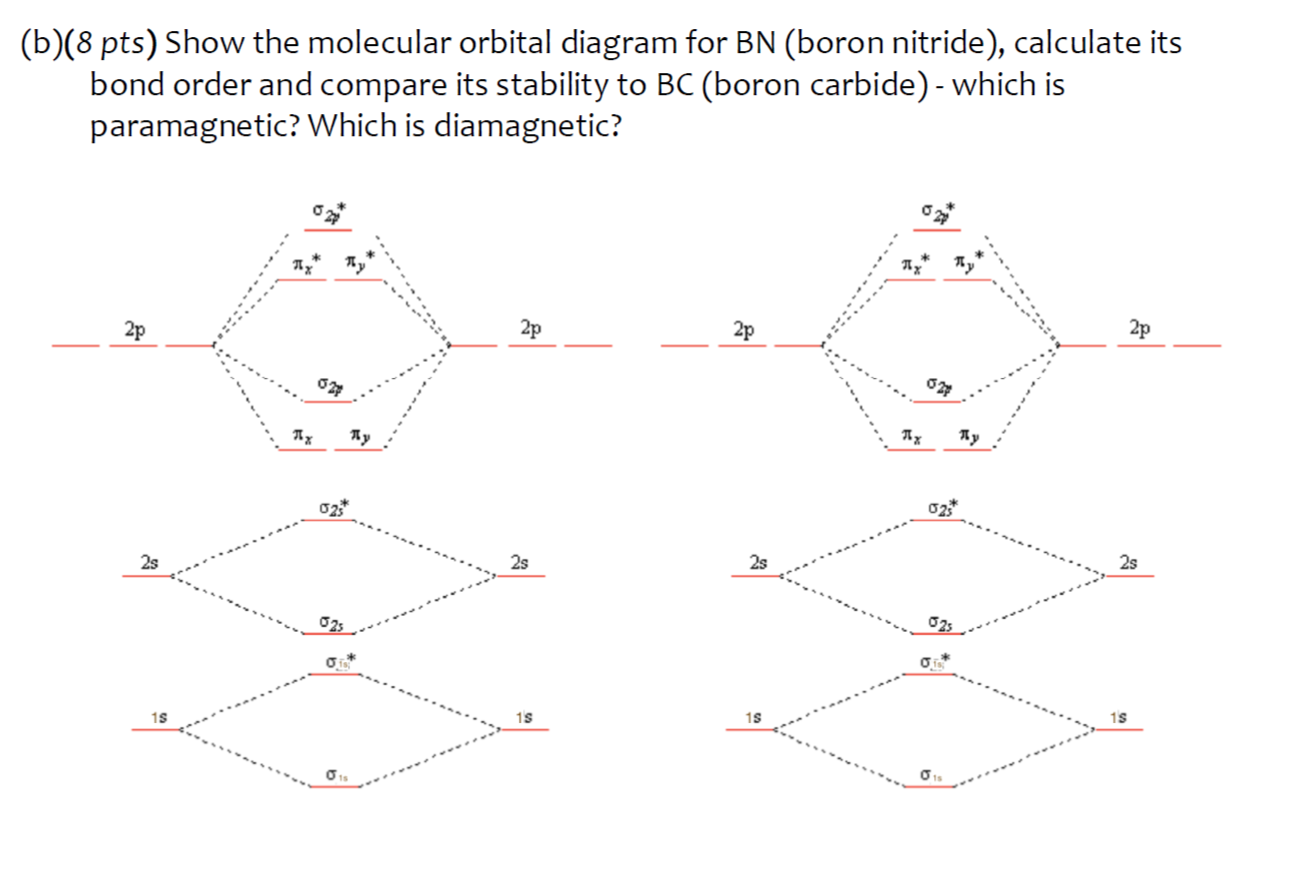
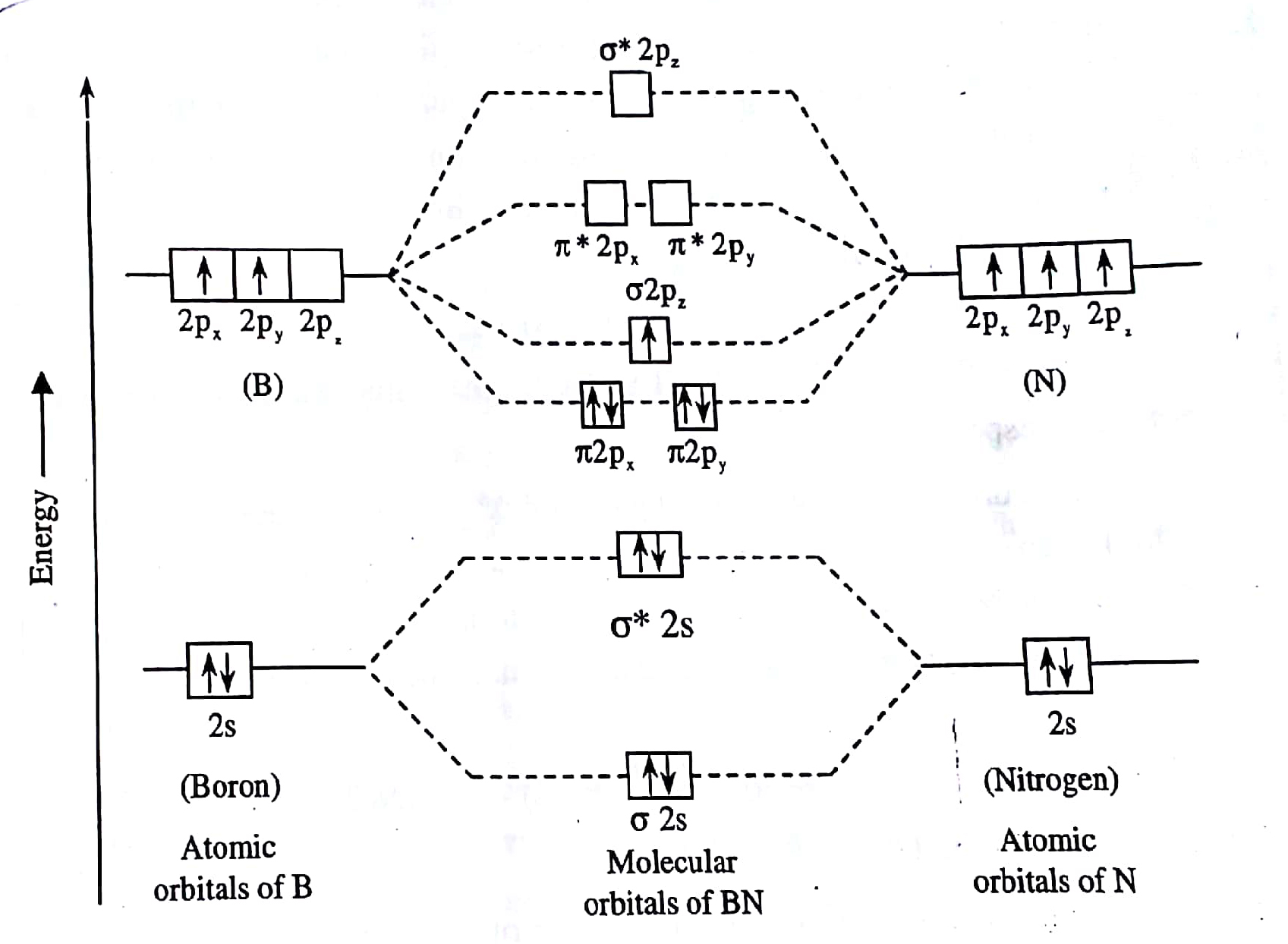
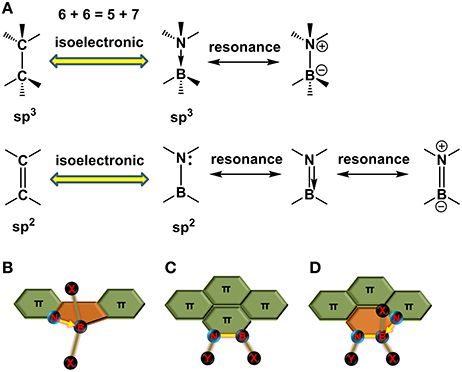
How to draw a BN molecular orbital diagram? | Socratic This is the general MO diagram you need to fill with the valence electrons of BN Boron has 3 valence electrons, and nitrogen has 5 valence electrons, this makes 8 orbital and another one on the πy orbital. So you end up with 2 unpaired electrons, and paramagnetism of the molecule is explained.
Energy level diagram for Molecular orbitals - Chemical Bonding and... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons. 2) If Nb < Na , the molecule is unstable because the antibonding influence is greater than the bonding influence, resulting in net force of repulsion.
8.4 Molecular Orbital Theory - Chemistry Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals.
Molecular Orbital Theory: Energy level diagram for molecular orbitals Molecular orbital theory was put forward by Hund and Mullikan in 1932. This theory is modern and more rational. This theory assume that in molecules This energy diagram for the molecular orbitals is shown in Fig.1 However, experimental evidence for oxygen and heavier diatomic molecules have...
Day 06 3 Molecular Orbital (MO) Theory for BN - YouTube Professor Chang Y. RyuDepartment of Chemistry and Chemical Biology, RPIGeneral Chemistry Discussion Problems.
Molecular Orbital Diagram Of Bn | Molecular, Molecular shapes... Sign in | Etsy. Laminated hand-drawn human anatomy study diagrams. Life Science & Biology with Mel and Gerdy.
Molecular Orbital Diagrams - Every Science The procedure for working out a molecular orbital of a general diatomic molecule is quite simple. We construct molecular orbitals using the available orbitals on the atoms. We then fill up the molecular orbitals, starting with the lowest in energy, until all the electrons in the species have been assigned to...












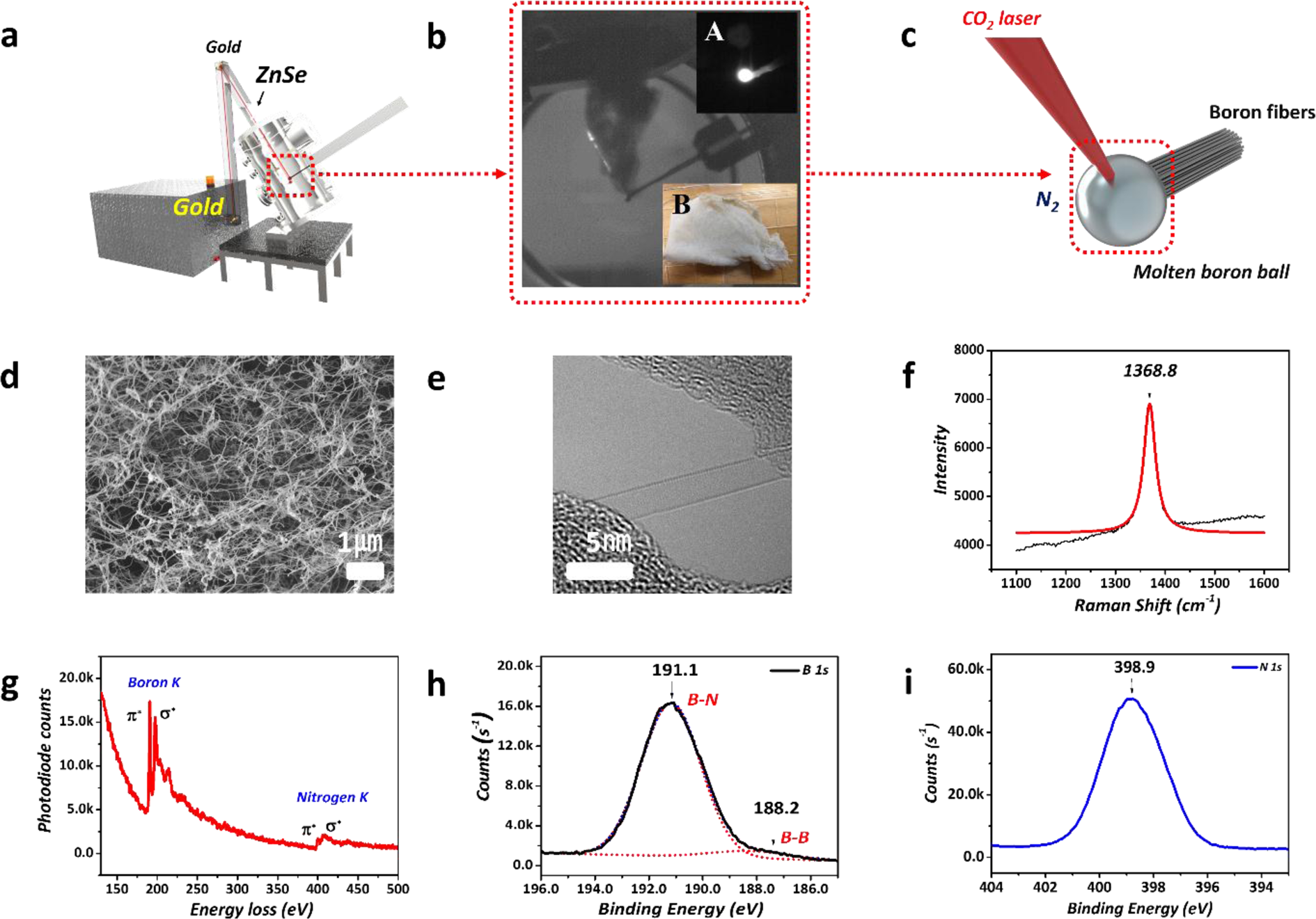




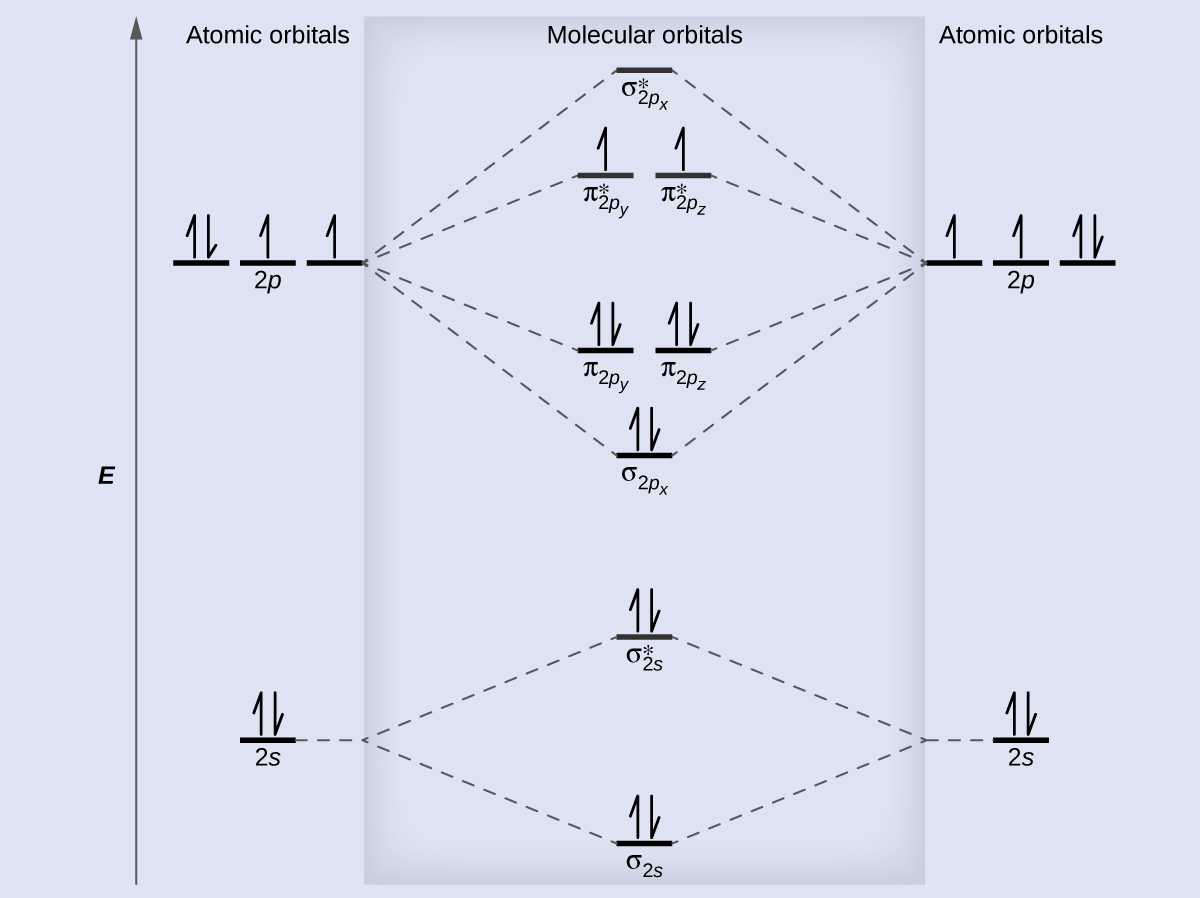
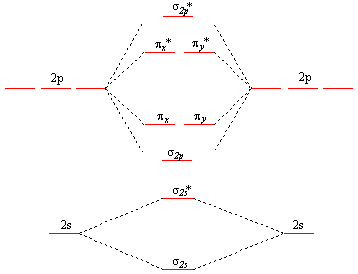

0 Response to "39 Bn Molecular Orbital Diagram"
Post a Comment