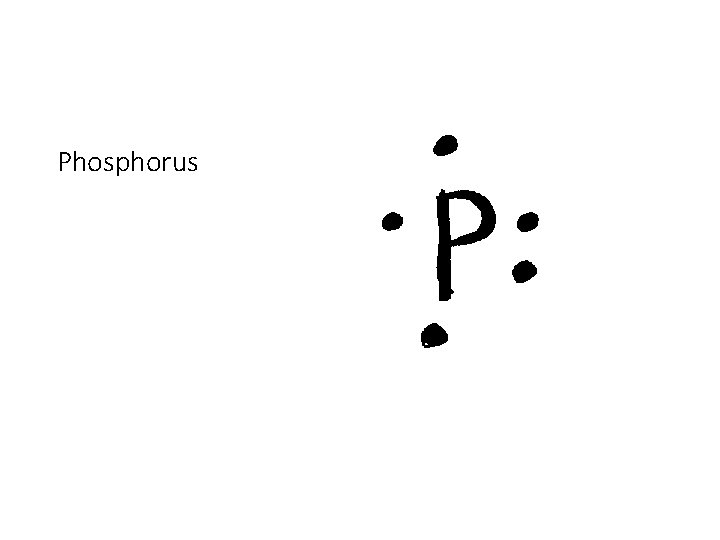
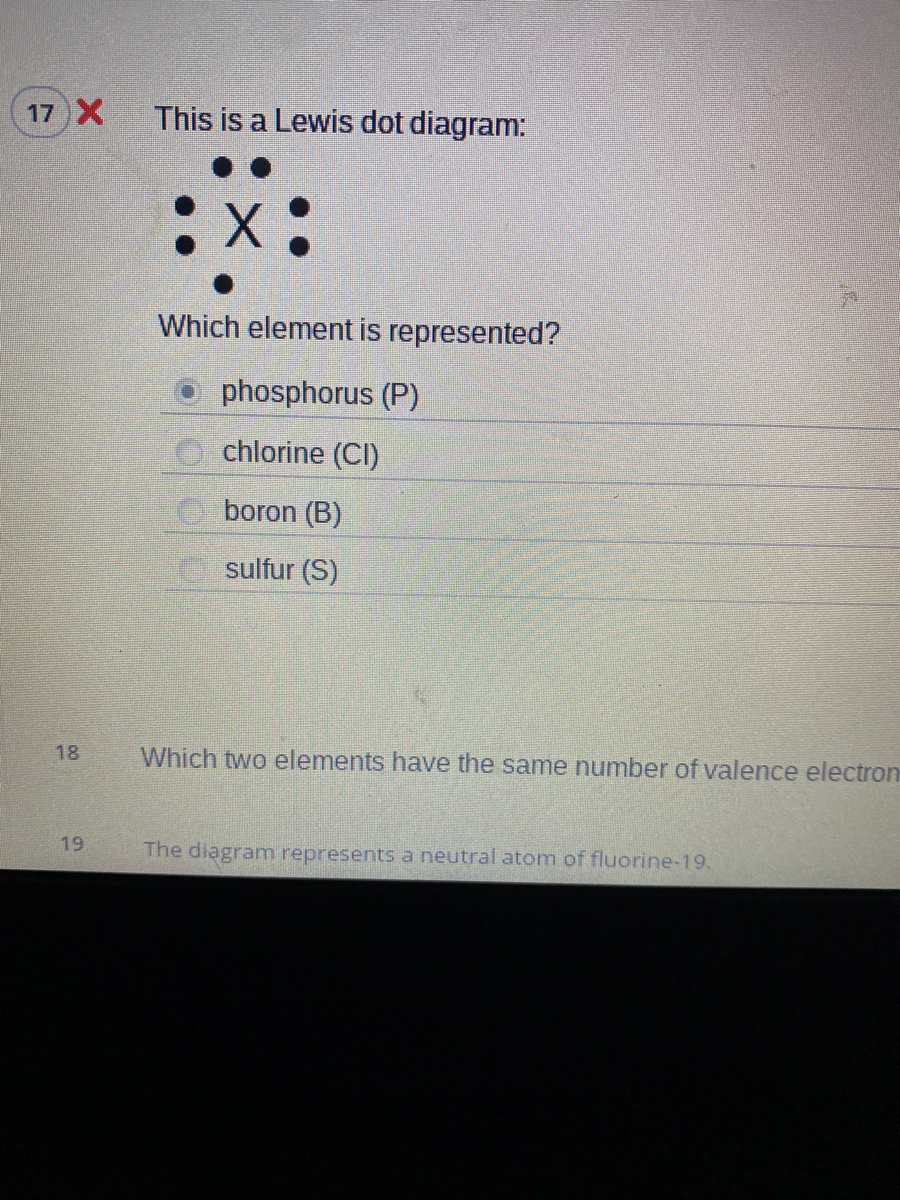
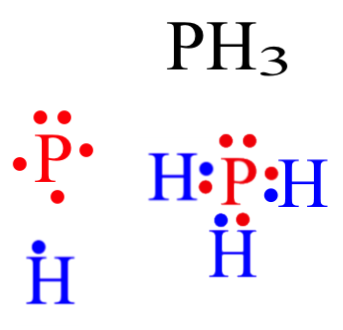
35 lewis dot diagram for phosphorus
what is the lewis dot structure for pbr3 - shapovmusic.com The key difference between Lewis dot symbol and Lewis structure is that Lewis dot symbol represents electrons in the outermost electron shell of an atom in a molecule whereas Lewis structure represents the structure of molecules using symbols for chemical elements and dot symbols. How to Draw the Lewis Dot Diagram of P (Phosphorus ... With elemental phosphorus (white phosphorus, P4) as a bonus.Check me out:
How to Draw the Lewis Dot Structure for P 3- (Phosphide ... A step-by-step explanation of how to draw the P3- Lewis Dot Structure.For the P3- Lewis structure use the periodic table to find the total number of valence ...

Lewis dot diagram for phosphorus
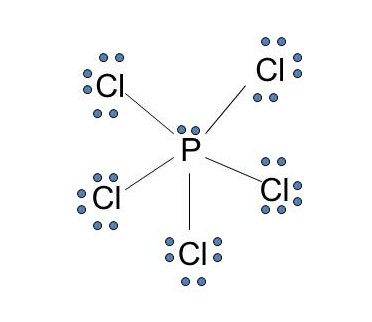
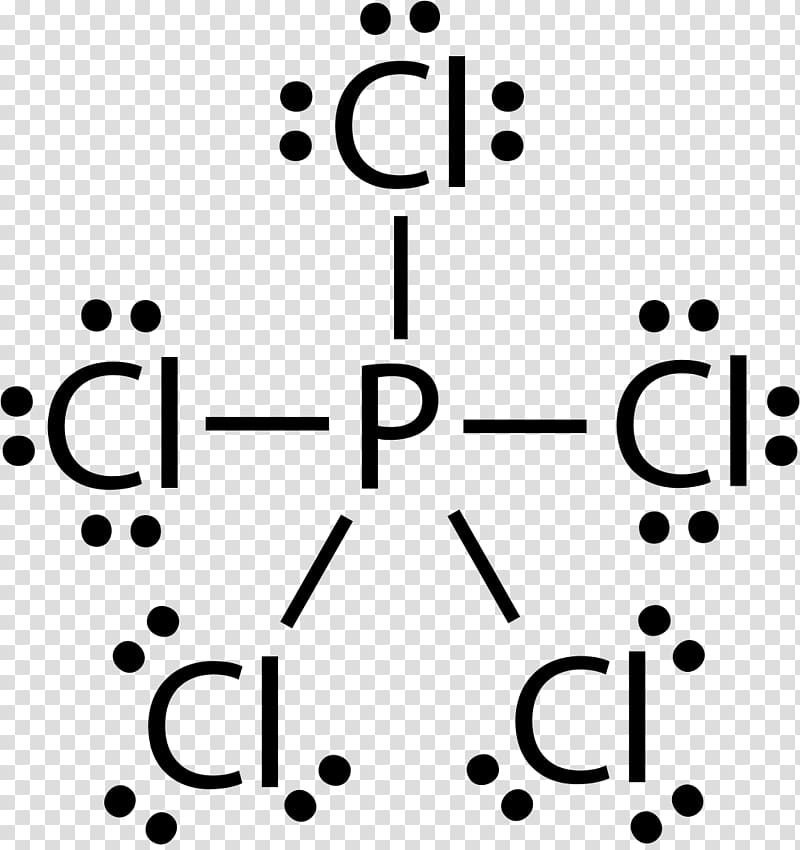
Solved a) Draw a valid lewis electron dot Structure for ... Expert Answer 100% (14 ratings) (A) There are 26 valence electrons to be used when drawing the Lewis dot structure for Phosphorous trichloride; 5 from phosphorus as it occurs in the 15th column of the Periodic Table and 7 from each of the chlorine atoms as chlorine occurs in the 17 … View the full answer Previous question Next question 6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. PCl3 (Phosphorus Trichloride) Lewis Structure PCl 3 (Phosphorus Trichloride) Lewis Structure. Phosphorus trichloride (PCl 3) contains three chlorine atoms and one phosphorus atoms. In PCl 3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Also, there is a lone pair on phosphorus atom. In this tutorial, we will learn how to draw the lewis structure of PCl 3 with all theories.
Lewis dot diagram for phosphorus. Lewis Dot Diagram Phosphorus - schematron.org Since there are 4 electron pairs around phosphorus, the geometry is based upon a tetrahedron, but since one of these electron pairs is a stereochemically active non-bonding pair, the. Lewis diagrams, also called electron-dot diagrams, are used to represent paired and unpaired valence (outer shell) electrons in an atom. Lewis dot diagram for phosphorus? - Answers the Lewis dot diagram is a table used for the elements, and it shows you how many valence electrons there are. Lewis Structure for PF5? It is Triangular Bipyrcamidal with a SP3d hybridization. dot... Lewis Dot Structure and VSEPR Model - sophat Lewis dot structure is a diagram that shows how atoms are bonding together and how many lone pairs of electrons are there in the molecule. For example, if we have an element of Phosphorus trifluoride (PF 3), we would start off by looking for the valence electrons. In this case, the valence electrons for Phosphorus and the three Fluorine is 5 ... PPTX AIM: How to write Lewis Dot Structures (Electron Dot ... Lewis Dot Structure (Electron Dot Structure) A Lewis dot structure is a quick and easy diagram that shows the valence electrons in an element.In a Lewis structure, the nucleus of the element is represented by its symbol. The valence electrons are represented by dots placed around the symbol in pairs.
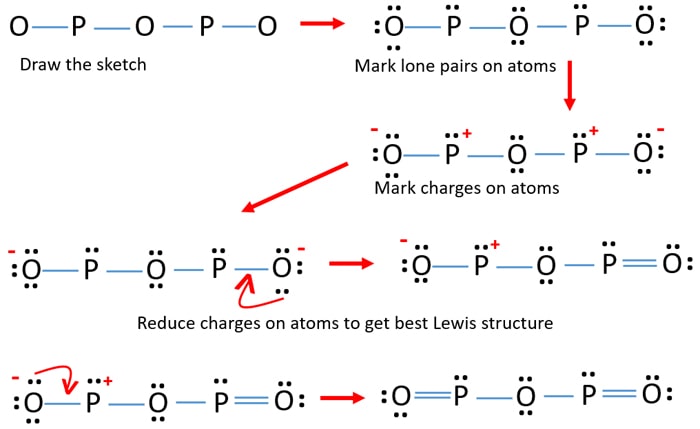
P2O5 (Phosphorus pentoxide) Lewis Structure P 2 O 5 (Phosphorus pentoxide) Lewis Structure. In the lewis structure of P 2 O 5, there are two elements; phosphorus and oxygen.Two phosphorus atoms are linked through an oxygen atom in the lewis structure of phosphorus pentoxide (P 2 O 5).All other oxygen atoms have made double bonds with phosphorus atoms. What is the Lewis dot structure for P? - FindAnyAnswer.com Beside above, what is the electron dot diagram for phosphorus? The electron dot or Lewis dot structure of P4,which is the constituent molecule of white phosphorus,can be easily drawn keeping in mind the facts that: 1)It has tetrahedral geometry. 2)Each P has 5 valence e-s and thus in P4 there are 5×4=20 valence e-s. Lewis Dot Diagram Magnesium And Phosphorus - Novocom.top lewis structure phosphorus atom dot electron draw electrons valence system dots satisfy wps facilitates represent uses socratic . Advertisiment. phosphorus quiz diagrams highlight weebly differences . ionic magnesium calcium lewis phosphide nitride sulfide . magnesium phosphate formula chloride . Lewis Dot Diagram For Phosphorus - schematron.org More information about the DOT ID/UN number and the guide number can be found at the Emergency Response Guidebook. Nov 22, · Best Answer: The Lewis Electron Dot Diagram for Phosphorous is P with 5 dots around it. There are 5 valence electrons, so this is how many dots are around. Each dot represents one electron.
Compare the Lewis dot structure of nitrogen and phosphorus ... Compare the Lewis dot structure of nitrogen and phosphorus and explain why you might expect these two atoms to exhibit similar bonding properties. Name one element that you would expect to exhibit bonding properties similar to boron. Explain.-----1.5 | Draw a Lewis dot structure for each of the following atoms: a) carbon b) oxygen c) Fluorine d) Hydrogen e) Bromine f) Sulfur g) Chlorine h ... Phosphorus Dot diagram - Summarized by Plex.page | Content ... In the PCl5 Lewis model, there are a total of 40 valence electrons. On the Periodic table, Phosphorous is in Period 3 when you draw the Lewis structure for PCl5: Remember that Phosphorous is in Period 3 on the Periodic table when you lay it out. How many dots would go around potassium in an electron dot diagram? Magnesium And Phosphorus Lewis Dot Structure - Novocom.top Magnesium And Phosphorus Lewis Dot Structure, Dot Cross Diagram Magnesium Fluoride YouTube, Step 4A: Place one lone pair on the center P atom to reach, What are valence electrons give example, phosphorus electrons Images Frompo 1 Phosphorus trifluoride (PF3) lewis dot structure ... According to the lewis dot structure of PF3, Phosphorous contains 1 lone pair and 3 bonded pair which is attached with three fluorine atoms. How does the PF3 lewis dot structure obey the octet rule? If an atom gets more or less than 8 electrons in an outermost shell then we can say that atom violates the octet.
Draw the Lewis dot diagram for phosphorus. | Study.com Normally, the Lewis dot diagram is utilized for the representation of the electron pairs, which usually consist of lone pair and valence electrons in it. The solid dot is the way to show the Lewis ...
PBr3 Lewis Structure, Molecular Geometry, Hybridization ... PBr3 Lewis Structure, Molecular Geometry, Hybridization and Polarity. PBr3 is a chemical formula for Phosphorus Tribromide. The molecule is made up of one Phosphorus atom and three Bromine atoms. It is a colourless liquid with a pungent odour. Like PF3 and PCl3, PBr3 also exhibits the properties of both Lewis Acid and Lewis Base.
Phosphorus Draw the Lewis dot structure for phosphorus ... Answer to: Phosphorus Draw the Lewis dot structure for phosphorus. Include all lone pairs of electrons. By signing up, you'll get thousands of...
What is the Lewis dot structure for BrCl? 2022 - Question ... What is the Lewis symbol for phosphorus? letter P So, to draw the Lewis Structure, begin by drawing the symbol for Phosphorus, the letter P. Next, Phosphorus has 5 valence electrons. So start with one dot on top, then one dot to the right, one dot on the bottom, one dot to the left, and another dot on top, next to the first one.
Lewis dot structures - xaktly.com Phosphorus pentachloride (PCl 5) is an exception to the octet rule. You can see the Lewis structure of PCl 3 in the practice problems below. Because a chlorine atom only needs one electron to complete its valence shell, it shares one and only one electron with phosphorus, so in PCl 5, phosphorus is surrounded by a total of ten electrons. It ...
What is the Lewis dot structure for PCl3? - handlebar ... We can clearly see from the lewis diagram that in PCl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. With that 2 lone pairs are present on the phosphorus atom. This concept very well explains the hybridization of PCl3 which is sp3. How is the phosphorus trichloride ( PCl3 ) made?
How would you draw a Lewis structure for an atom that has ... So, to draw the Lewis Structure, begin by drawing the symbol for Phosphorus, the letter P. Next, Phosphorus has 5 valence electrons. So start with one dot on top, then one dot to the right, one dot on the bottom, one dot to the left, and another dot on top, next to the first one.
Solved Phosphorus Draw the Lewis dot structure for | Chegg.com See the answer See the answer done loading. Phosphorus. Draw the Lewis dot structure for phosphorus. Include all lone pairs of electrons. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (8 ratings)
Lewis Dot Diagram Phosphorus - wiringall.com Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. Nov 22, · Best Answer: The Lewis Electron Dot Diagram for Phosphorous is P with 5 dots around it. There are 5 valence electrons, so this is how many dots are around. Each dot represents one electron.
PCl3 (Phosphorus Trichloride) Lewis Structure PCl 3 (Phosphorus Trichloride) Lewis Structure. Phosphorus trichloride (PCl 3) contains three chlorine atoms and one phosphorus atoms. In PCl 3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Also, there is a lone pair on phosphorus atom. In this tutorial, we will learn how to draw the lewis structure of PCl 3 with all theories.
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
Solved a) Draw a valid lewis electron dot Structure for ... Expert Answer 100% (14 ratings) (A) There are 26 valence electrons to be used when drawing the Lewis dot structure for Phosphorous trichloride; 5 from phosphorus as it occurs in the 15th column of the Periodic Table and 7 from each of the chlorine atoms as chlorine occurs in the 17 … View the full answer Previous question Next question

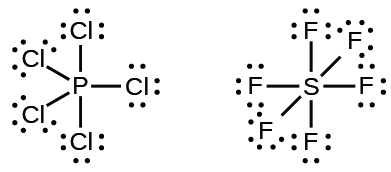








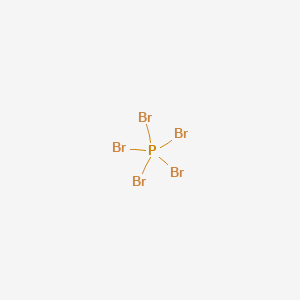








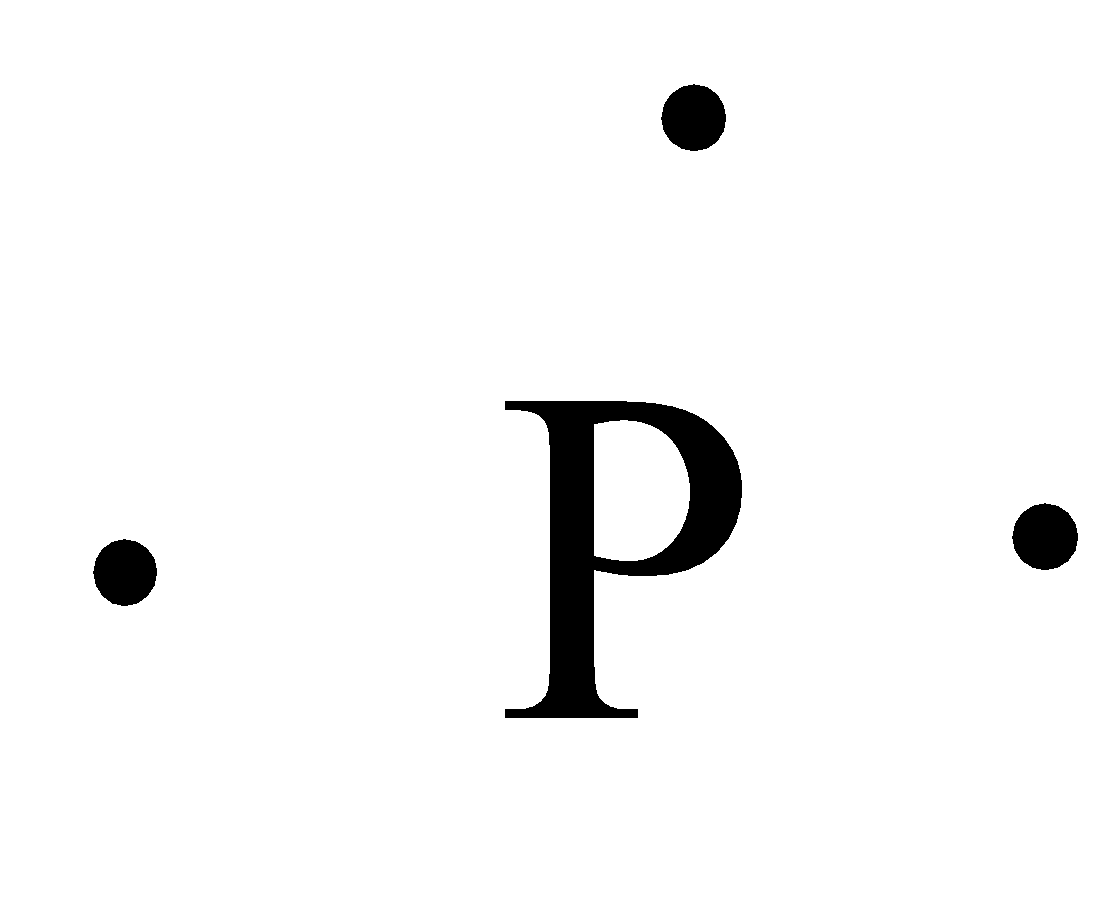


0 Response to "35 lewis dot diagram for phosphorus"
Post a Comment