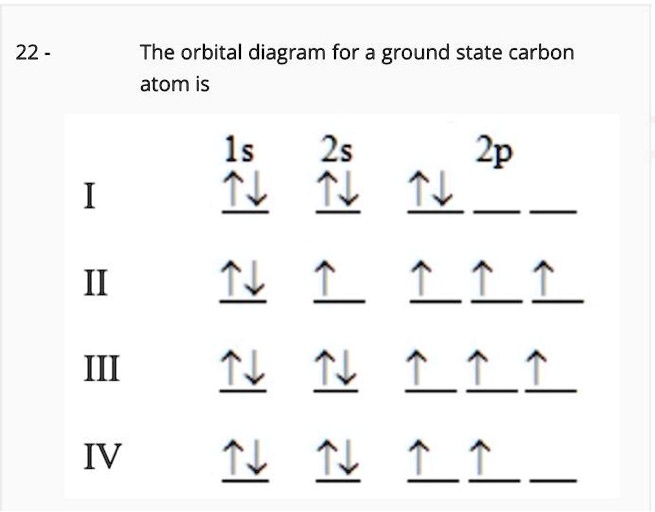
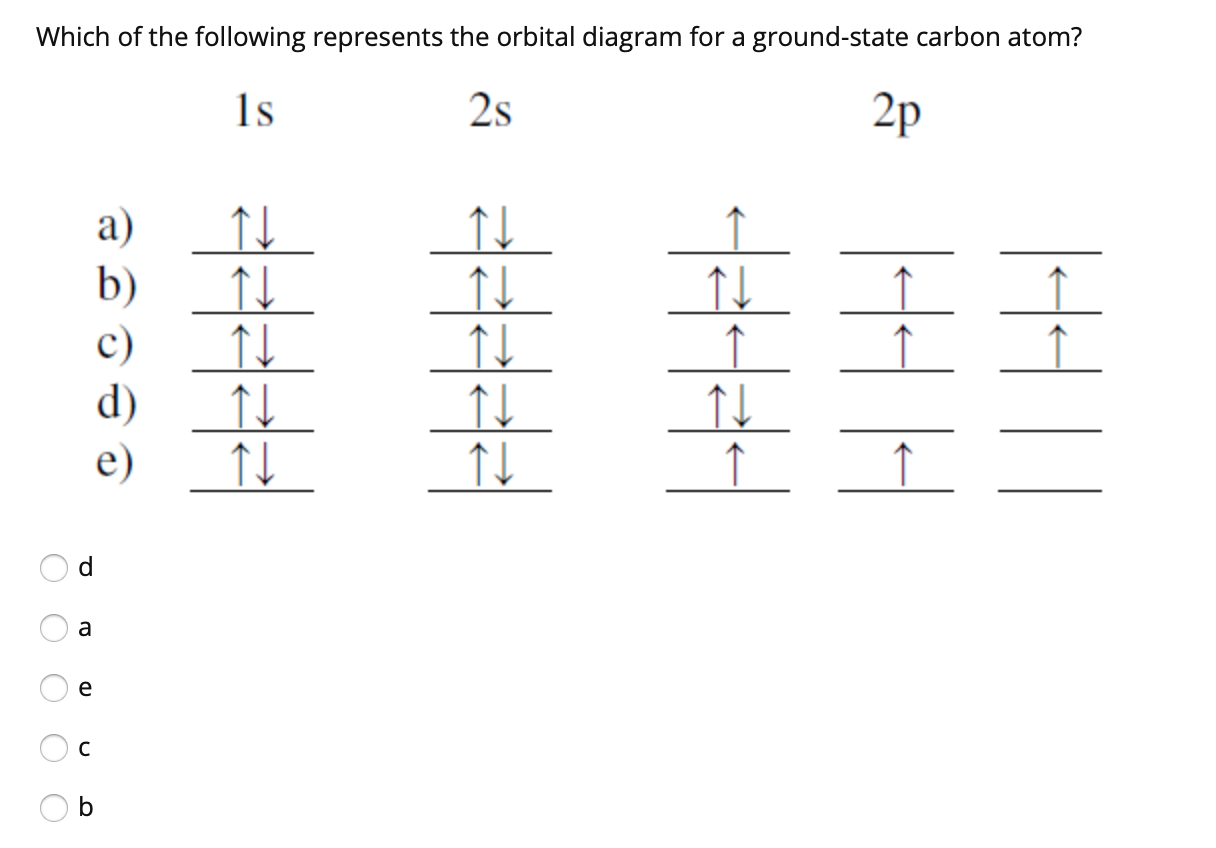
35 the orbital diagram for a ground state carbon atom is
Orbital Diagram Of Carbon Before Sp3 Hybridization The ground state configuration of carbon is 1s 2 2s 2 2px 1 2py 1. The p orbitals are equal in energy and said to be degenerate. The two singly occupied p orbitals can be utilized for bonding to give methylene CH 2, an unstable free radical (Figure 3). HW #3 Flashcards - Quizlet orbital node crevice pit. Node. The ground-state electron configuration of a calcium atom is [Ne]3s2 [Ar]4s13d1 [Ne]3s23p6 [Ar]4s2 [Ar]3d2 ... The orbital diagram for a ground state carbon atom is 1s 2s 2p A ↿⇂ ↿⇂ ↿⇂ . B ↿⇂ ↿ . ↿ . ↿ . ↿ C ↿⇂ ↿⇂ ↿ . ↿ . ↿
What is the orbital diagram for a ground-state nitrogen ... A nitrogen atom has 3 orbitals; the 1s orbital, the 2s orbital, and the 2p orbital. In this case, the 2s and 2p orbitals are the valence orbitals, as they have the electrons with the most energy.
The orbital diagram for a ground state carbon atom is
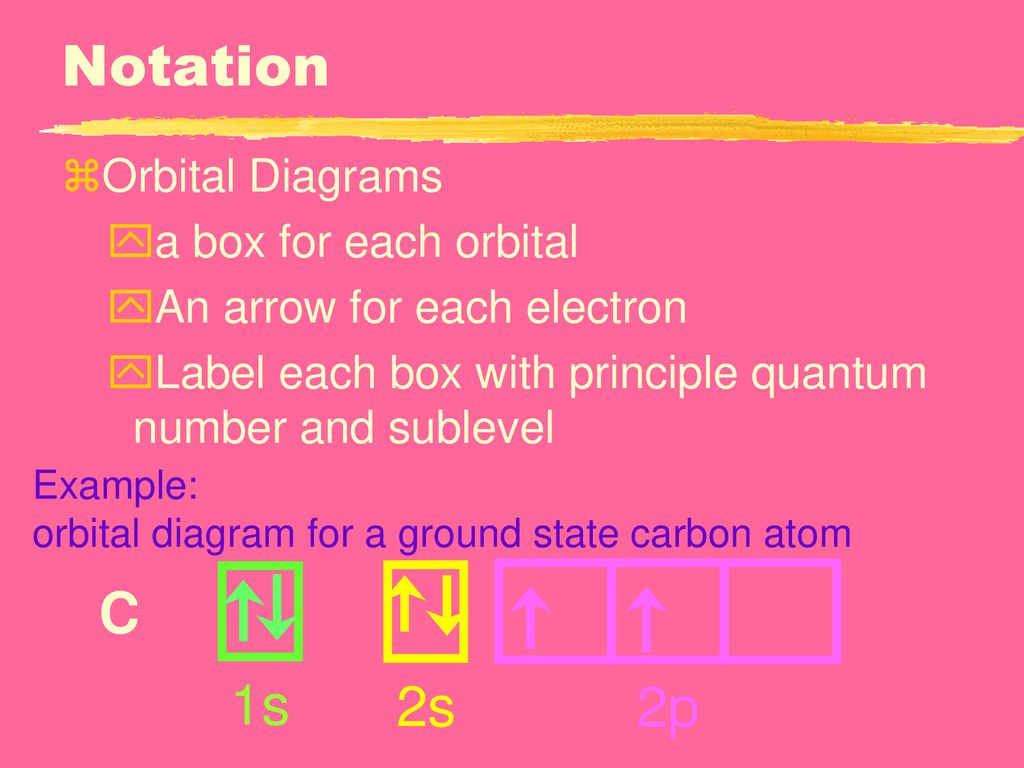
Orbital Diagram For Carbon (C) | Carbon Electron Configuration The element carbon has 6 electrons in total and one of the main things that many users might not know is the symbol by which it is represented. The symbol of carbon is written as 6. Carbon Electron Dot Diagram. If we talked about the electronic configuration of the element then, carbon is an element whose electronic configuration is given as 1s ... Hund's Rule and Orbital Filling Diagrams | Chemistry for ... Orbital Filling Diagrams. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. How do you write the orbital diagram for carbon? | Socratic The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with opposite spins (Pauli's exclusion principle). In a neutral carbon atom, the "1s" sublevel has one orbital with two electrons with opposite spins, represented by the arrows pointing in opposite directions.
The orbital diagram for a ground state carbon atom is. D3.3 Orbital Energy Level Diagrams - Chemistry 109 Fall 2021 For example, a ground state boron atom has this orbital energy level diagram: As illustrated by the boron example, an orbital diagram includes all orbitals in all subshells within a partially occupied shell, even if some orbitals are unoccupied. Orbital Diagram For Beryllium Be 's ground-state electron configuration is the. Electron configurations and orbital diagrams can be determined by applying the An atom of the alkaline earth metal beryllium, with an atomic. Since the 2s orbital is completely filled, a new type of orbital must be of one more atom, carbon, with the aid of the color-coded diagrams. 15 The orbital diagram for a ground state nitrogen atom is ... 15 The orbital diagram for a ground state nitrogen atom is 15 A D B A C C D B 3 from CHEM 1110 at CUNY New York City College of Technology Chapter 7: Quantum Theory and the Electronic Structure of ... The orbital diagram for a ground-state oxygen atom is 14. The orbital diagram for a ground state carbon atom is 15. Which ground-state atom has an electron configuration described by the following orbital diagram? A) phosphorus B) germanium C) selenium D) tellurium E) none of these 16.
Carbon(C) electron configuration and orbital diagram In the carbon (C) ground-state electron configuration, the two electrons of the 3p orbital are located in the p x, p y sub-orbitals and the spin of the two electrons is the same. Carbon (C) excited state electron configuration and orbital diagram Then correct electron configuration of carbon in ground state will be 1s 2 2s 2 2p x1 2p y1. Electron Configurations and Orbital Box Diagrams ... The electron configuration for carbon is 1s 2 2s 2 2p 2. An orbital box diagram can be written as well. Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin-one arrow pointing up and the other one pointing down. What is the orbital notation of carbon? - FindAnyAnswer.com Carbon has 6 protons and electrons, so it has 2 in the 1S orbital, 2 in the 2S orbital, and 2 in the 1P orbital. This is often expressed as [HE]2S2 2Ps, because it has the same configuration as helium plus 4 additional electrons whose positions are shown after the bracketed element. Click to see full answer How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Answered: The orbital diagram for a ground state… | bartleby The orbital diagram for a ground state carbon atom is 1s 2s 2p A. T TI B. ÎN Î ÎÎÎ c. 似 11个 D. TL ÎL Î Î_ check_circle Expert Answer Want to see the step-by-step answer? See Answer Check out a sample Q&A here. Want to see this answer and more? Experts are waiting 24/7 to provide step-by-step solutions in as fast as 30 minutes!* See Answer Oxygen(O) electron configuration and orbital diagram The ability of one atom of an element to join another atom during the formation of a molecule is called valency. The number of unpaired electrons in the last orbit of an element is the valency of that element. The correct electron configuration of oxygen in ground state will be 1s 2 2s 2 2p x 2 2p y 1 2p z 1. This electron configuration shows that there are two unpaired electrons in the last orbit of oxygen. Carbon Orbital diagram, Electron configuration, and Valence ... The orbital diagram for Carbon is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The Carbon orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, and the rest two electrons in 2p orbital. Orbital diagram for a ground-state electron configuration of Carbon atom is shown below- Solved The orbital diagram for a ground state carbon atom is ... The orbital diagram for a ground state carbon atom is 1s 2s A) A B) B C) D D) C Calculate the wavelength of the light emitted by a hydrogen atom during a transition of its electron from the n-4 to the n-1 principal energy level. Recall that for hydrogen En -一2.18 × 10-18 J (1/n2) A) 0.612 nm B) 97.2 nm C) 82.6 nm D) 365 nm E) 6.8 × 10-18 nm.
What is the orbital diagram for carbon? - MSI Carbon has 6 protons and electrons, so it has 2 in the 1S orbital, 2 in the 2S orbital, and 2 in the 1P orbital. This is often expressed as [HE]2S2 2Ps, because it has the same configuration as helium plus 4 additional electrons whose positions are shown after the bracketed element.
Chapter 7: Quantum Theory and the Electronic Structure of ... The orbital diagram for a ground-state nitrogen atom is. 13. The orbital diagram for a ground-state oxygen atom is. 14. The orbital diagram for a ground state carbon atom is. 15. Which ground-state atom has an electron configuration described by the following orbital diagram? A) phosphorus B) germanium C) selenium D) tellurium E) none of these ...
What is the orbital diagram for a ground state carbon atom ... The ground state is 1s^2 2s^2 2p^2. In the explanation below, I show a common means of diagramming this. Using arrows to show the spin orientation of each electron, the orbital diagram is often shown this way: The single electrons in the two p-orbitals is in accordance with Hund's Rule.
Orbital Diagram For Germanium - schematron.org 0 Comments. on Orbital Diagram For Germanium. orbital. Because an electron can have either one of two spins, any orbital can hold a maximum of four . The orbital diagram for germanium is. 1s. 2s. 2p. 3s. Oxidation States, +4,2. Electrons Per Shell, 2 8 18 4. Electron Configuration, [Ar] 3d10 4s2 4p2. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2.
6.4 Electronic Structure of Atoms (Electron Configurations ... electronic structure of an atom in its ground state given as a listing of the orbitals occupied by the electrons. Hund's rule. every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied, and all electrons in singly occupied orbitals have the same spin. orbital diagram.
The orbital diagram for a ground-state carbon atom is Recent Posts. Reba's father, George, is 84 years old. He had his first stroke in his early seventies or about 10 years ago. Since then, he has had TIA's transient ischemic attacks)
P a g e 7 15 The orbital diagram for a ground state ... P a g e 7 15 The orbital diagram for a ground state nitrogen atom is 1s 2 2s 2 from PHYS 240 at San Francisco State University
Ground State Electron Configuration of an Atom | Rules, Terms ... Jan 27, 2022 · In orbital diagrams, this is signified using up and down arrows. In the carbon orbital diagram, the 1s and 2s subshells have two electrons. One is designated with an up arrow, and one is designated...
What Is The Orbital Diagram Of Oxygen? By Hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Draw an orbital diagram for nitrogen, Z = 7. ১০ ফেব, ২০২১
PDF Electron Configurations C1YvM - Weebly 1. Which orbital diagram represents a boron atom in the ground state? 2. Based on the charge of an electron, why would electrons prefer to be in different orbitals of the same sublevel if possible? 3. Match the definition to the correct 'rule'. _____A. Rules of Aufbau I. Electrons in orbitals must have opposite spins.
Exam 3 Study Guide Flashcards | Quizlet A possible set of quantum numbers for the last electron added to complete an atom of gallium (Ga) in its ground state is. n l ml ms. 4 1 0 +1/2. The orbital diagram for a ground-state nitrogen atom is. 1s 2s 2p. The orbital diagram for a ground state carbon atom is. 1s 2s 2p.
How do you write the orbital diagram for carbon? | Socratic The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with opposite spins (Pauli's exclusion principle). In a neutral carbon atom, the "1s" sublevel has one orbital with two electrons with opposite spins, represented by the arrows pointing in opposite directions.
Hund's Rule and Orbital Filling Diagrams | Chemistry for ... Orbital Filling Diagrams. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally.
Orbital Diagram For Carbon (C) | Carbon Electron Configuration The element carbon has 6 electrons in total and one of the main things that many users might not know is the symbol by which it is represented. The symbol of carbon is written as 6. Carbon Electron Dot Diagram. If we talked about the electronic configuration of the element then, carbon is an element whose electronic configuration is given as 1s ...

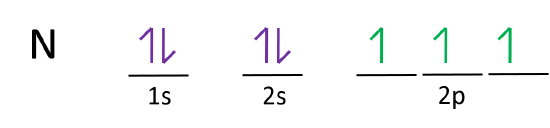




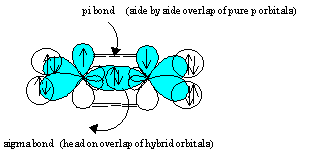
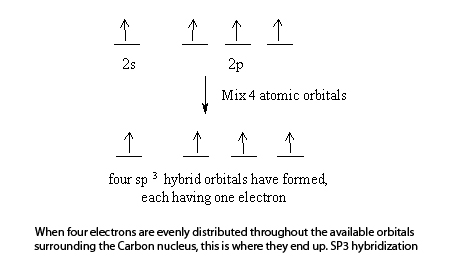



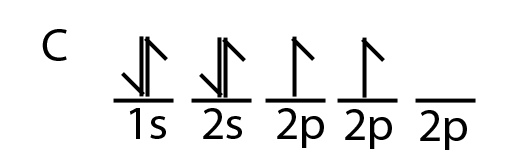

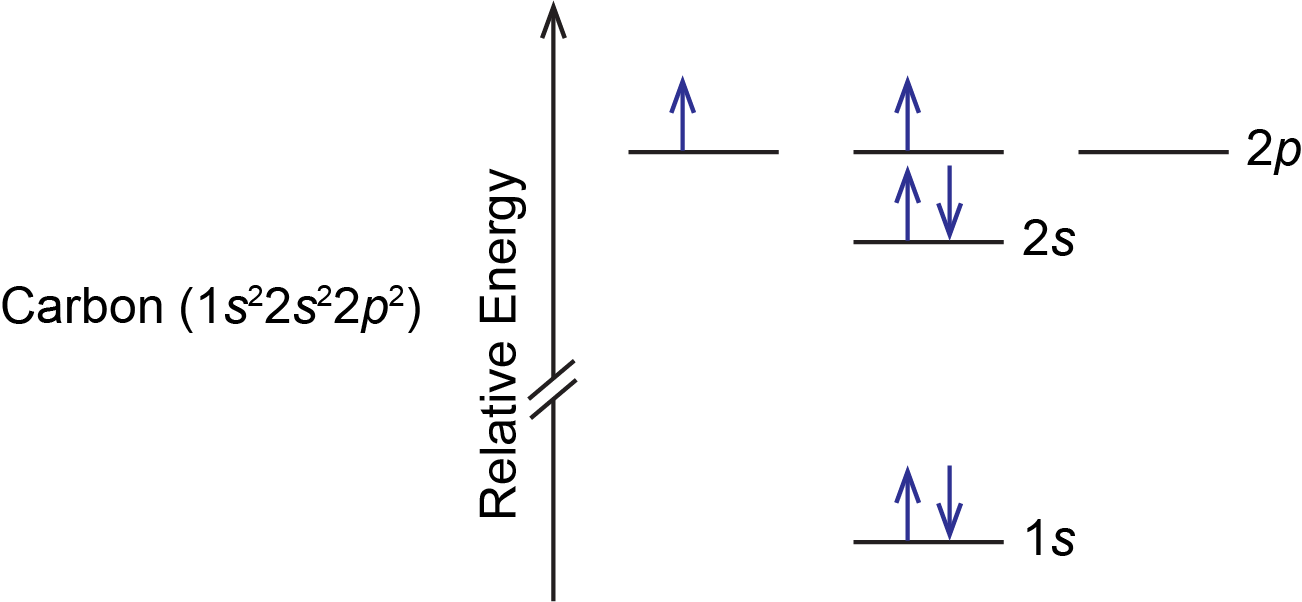











0 Response to "35 the orbital diagram for a ground state carbon atom is"
Post a Comment