37 trigonal bipyramidal mo diagram
Considering s-bonding only, in the MO diagram of a metal ... CSIR NET; Chemical Sciences; Considering s-bonding only, in the MO diagram of a metal complex with trigonal bipyramidal Lecture 14: d-Orbital Splitting and Trigonal Bipyramidal ... G4071 - Fall 2021Jonathan Owen, Columbia Unversity
PDF IMF Unit - Lewis Structure, VSEPR Theory, VB Hybridization ... IMF Unit - Lewis Structure, VSEPR Theory, VB Hybridization, MO Theory Fill in the chart below. Molecule N 2 O 2 HF SeCl 4 Lewis Structure VSEPR: Electronic geometry of central atom(s)? Linear Trigonal Planar H: Linear F: Tetrahedral Trigonal Bipyramidal VSEPR: Molecular
Trigonal bipyramidal mo diagram
(Color online) (a) MO 5 trigonal bipyramid and schematic ... Download scientific diagram | (Color online) (a) MO 5 trigonal bipyramid and schematic energy levels of the 3d bands of the TM ion in trigonal bipyramidal coordination, and (b) schematic energy ... Construct a plausible Molecular Orbital (MO) energy diagram ... In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation PDF MO Diagrams for More Complex Molecules MO Theory • MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection.
Trigonal bipyramidal mo diagram. SF4 Lewis Structure, Molecular Geometry, Hybridization ... SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. SF4 or sulfur tetrafluoride is a compound that has a distinct odor of sulfur or rotten eggs. This compound is generally identified as being a colorless gas. The molecular weight of this compound is calculated to be 108.6 g/mol. SF4's boiling and melting points are -38 ... Trigonal Bipyramidal Molecular Geometry - Chemistry LibreTexts August 21, 2020 - NOTES: This molecule is made up of 5 sp3d hybrid orbitals. Three orbitals are arranged around the equator of the molecule with bond angles of 120o. Two orbitals are arranged along the vertical axis at 90o from the equatorial orbitals. The shape of the orbitals is trigonal bipyramidal. chemistry 1a Chapter 10 Flashcards | Quizlet trigonal bipyramidal geometry. Positions above/below central atom: axial positions. Positions in the same base plane as the central atom: equatorial positions. ... Use the molecular orbital diagram shown to determine which of the following are paramagnetic. A) O2 2⁺ ... Molecular orbital energy level diagrams for: (a) a high-spin ... Download scientific diagram | Molecular orbital energy level diagrams for: (a) a high-spin trigonal bipyramidal complex of Mn(II) and (b) a high-spin octahedral complex of Mn(II), considering in both cases only the contributions from the ligand. The molecular orbital stabilization energy (MOSE) ...
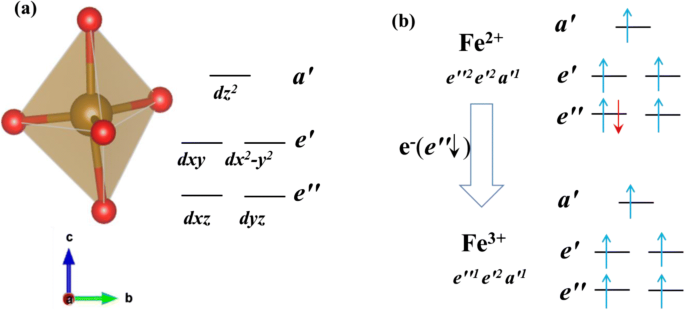
PDF Ligand Field Theory Quadratic Pyramidal Trigonal Bipyramidal Octahedral. Rubredoxin 3,4-PCD Tyrosine Hydroxylase Lipoxygenase Tetrahedral Trigonal ... Tanabe-Sugano Diagrams ... MO Theory of ML 6 Complexes Key Points: ‣Filled ligand orbitals are lower in Chapter 6 – Molecular Structure In this section, we discuss the shapes of molecules in which the central atom has five and six electron regions. • Name the shapes adopted around atoms with five and six electron regions. ... Five groups around a central atom adopt a trigonal bipyramidal structure, which contains two distinctly ... Shown below is a partially completed MO diagram for ... Shown below is a partially completed MO diagram for trigonal bipyramidal D3h trans-FeL 2 (CO) 3 in which the electronic structure has been derived from the interaction of the Molecular orbitals of trigonal bipyramidal FeL 5 (L= neutral sigma donor only ligand) with the pi-acceptor SALCs of three carbon monoxide ligands arranged in a triangle.. Please use D3h irreducible representation chart ... PDF Chem 673, Problem Set 5 Due Tuesday, December 2, 2008 (1) tbp (3) Construct a d2 state correlation diagram for a trigonal bipyramidal environment that is analogous to the diagrams given in Cotton in Figures 9.3 and 9.4. Use your MO energy diagram from problem #1a to help get the strong-field limit side of your diagram. [In the problem that follows, some of your answers to later parts will
d orbital splitting in Trigonal Pyramidal Field | VIPEr correctly fill in a d orbital splitting MO diagram to determine the ground state, applying Aufbau, Hunds Rules and Pauli exclusion principle, for either high or low spin and for any d electron count provided. ... The inorganic organic model kit containing a trigonal bipyramidal central atom could prove useful. ... 8.2 Hybrid Atomic Orbitals – Chemistry In a molecule of phosphorus pentachloride, PCl5, there are five P–Cl bonds (thus five pairs of valence electrons around the phosphorus atom) directed toward the corners of a trigonal bipyramid. We use the 3s orbital, the three 3p orbitals, and one of the 3d orbitals to form the set of five ... Hybrid Atomic Orbitals | Chemistry I In a molecule of phosphorus pentachloride, PCl5, there are five P–Cl bonds (thus five pairs of valence electrons around the phosphorus atom) directed toward the corners of a trigonal bipyramid. We use the 3s orbital, the three 3p orbitals, and one of the 3d orbitals to form the set of five ... Part A Complete the atomic orbital AO and molecular ... ANSWER: Bond angles Help Reset trigonal bipyramidal T-shaped seesaw square planar bent square pyramidal trigonal pyramidal tetrahedral trigonal planar linear octahedral 4/22/2021 VSEPR and MO Diagrams Workshop Activity - April 22nd 30/49 The types of electron groups not only affect the electron and molecular geometries but have differing ...
5. For this problem, you will generate an MO diagram - Chegg For this problem, you will generate an MO diagram for a trigonal bipyramidal transition metal complex, ML5 a. Determine the symmetries of the metal ion 4s, 4p, and 3d orbitals. b. Next, use the method discussed in class to generate all of the ligand SALCs for 0-only interaction C. Write out the wavefunctions for these σ-only ligand SALCs. d.
(PDF) Electronic Stabilization of Trigonal Bipyramidal ... Electronic Stabilization of Trigonal Bipyramidal Clusters: the Role of the Sn(II) Ions in [Pt 5 (CO) 5 {Cl 2 Sn(μ-OR)SnCl 2 } 3 ] 3- (R = H, Me, Et, i Pr) Inorganic Chemistry, 2011. Carlo Mealli. Andrea Ienco. Gabriele Manca. Giuliano Longoni. Download Download PDF.
Chem Flashcards - Quizlet D) all of the above bonds are the same strength. A. Identify the shortest bond. A) single covalent bond. B) double covalent bond. C) triple covalent bond. D) all of the above bonds are the same length. C. 16) Identify the compound with the smallest dipole moment in the gas phase.
PDF Hybridization and Molecular Orbital (MO) Theory Trigonal Bipyramidal Electronic Geometry: AB 5, AB 4U, AB 3U2, and AB 2U3 Some examples of molecules with this geometry are: PF 5, AsF 5, PCl 5, etc. • These molecules are examples of central atoms with five bonding pairs of electrons. The electronic and molecular geometries are the same. • Molecules are trigonal bipyramidal and nonpolar
PDF Trigonal Planar Molecules 3 trigonal planar 180 120 4 tetrahedral / pyramidal / bent 5 trigonal bipyramidal (and derivatives) 109.5 90 and 120 6 octahedral (and derivatives) Groups around central atom Shape Bond angle(s) in degrees 90 VSEPR shapes: VSEPR shapes "Groups" can be either BONDS or LONE PAIRS!
PDF Polyatomic Molecular Orbital Theory - La Salle University 13 Molecular Orbital Theory - BH 3 B H H H z y x The BH 3 molecule exists in the gas phase, but dimerizes to B 2H6 (which we will look at a bit later) 2 BH 3 B2H6 The BH 3 molecule is trigonal planar and we will make the C 3 principal axis of symmetry the z axis, with the x and y axes in the plane
A linear B trigonal planar C tetrahedral D trigonal ... E ) eg=trigonal planar , mg=trigonal planar , sp 2 , and nonpolar 22 ) Use a molecular orbital diagram shown to determine which of the following is / are paramagnetic . A ) O 2 2 ⁻
Transition Metal Pentacoordination - Roald Hoffmann A unified molecular orbital treatment of pentacoordinate transition metal complexes for the D3h trigonal-bipyramidal and the C4v square-pyramidal geometries ...
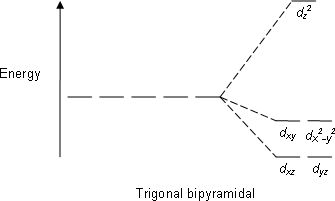
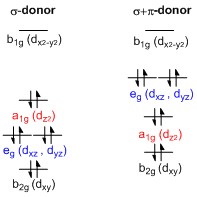
PDF D-orbital splitting diagrams - University of California ... D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns: 1. Octahedral 2. Tetrahedral 3. Trigonal bipyramidal 4. Square pyramidal d z2x2-y d xy d yzxz 5. Square planar d z2x2-y d xy d yzxz d z2 d x2-yxy d yz d xz d z2 d x2-y2 d xy d yz d ...
PDF 5.04 Principles of Inorganic Chemistry II Fall 2008 For ... The octahedral molecular orbital (MO) diagram provides the starting point for the construction of the electronic structure of several metal complexes. But not all complexes are conveniently referenced to octahedral geometry. Other important geometries include tetrahedral, square planar, trigonal bipyramidal and pyramidal.
CHEM 2303 - Supplementary Problems - Acadia U a. sp3d: Trigonal Bipyramidal Homonuclear Diatomic MO Diagrams Question 1 The Lewis Structure predicts either three bonds or one bond and a lone pair of electrons on each boron atom. The MO Diagram predicts two lone pairs of electrons and a lone electron on each boron atom - no bonds. The MO Diagram predicts a paramagentic molecule.
VSEPR theory - Wikipedia Lone pair–lone pair (lp–lp) ... decisions about overall geometry when 2 or more non-equivalent positions are possible. For instance, when 5 valence electron pairs surround a central atom, they adopt a trigonal bipyramidal molecular geometry with two collinear axial positions ...
What does the crystal field splitting diagram for trigonal ... The most basic crystal field argument includes point-symmetric charges approaching the central metal in a way as the ligands would. Then, any orbitals that are symmetry-equivalent will end up at the same energy, and depending on how much these point towards the point-symmetric approaching charges they will be raised or lowered.
4.6: Hybridization using d Orbitals - Chemistry LibreTexts August 5, 2021 - In these cases, the central atom ... five or more bonded atoms (as in PF5 and SF6). Using the ns orbital, all three np orbitals, and one (n − 1)d orbital gives a set of five sp3d hybrid orbitals that point toward the vertices of a trigonal bipyramid (part (a) in Figure ...
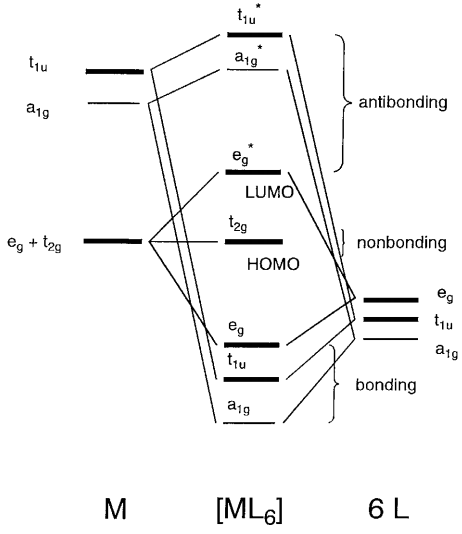
d-Metal Complexes - University of Massachusetts Lowell A molecular orbital diagram which estimates the energies of the bonding (show above) antibonding and non-bonding orbitals is shown below. Since there is a large disparity in energy between the ligand orbitals and the metal orbitals, the lower lying molecular orbitals in the diagram are essentially ligand orbitals.
PDF (c) trigonal bipyramid - University of Massachusetts Boston -35-(b) The P.E.S. results are consistent with the MO scheme. Only the Bn(x) MO is strictly nonbonding, while the F(z) MO is weakly bonding, as indicated by the vibrational fine structureon its P.E.S. band. (c) Rather than two lone pairs in approximately sp3 hybrids, the MO scheme suggests a single region of electron density protruding from the back side of the molecule.
PDF Inorganic Chemistry with Doc M. Day 17. Transition Metals ... 2. The MO energy diagram and Δo 1. Octahedral transition metal complexes utilize s, p and d-orbitals in their bonding. For a first row transition metal, these are the 3d, 4s and 4p orbitals (the valence orbitals). Here we will create a molecular orbital diagram that could be used for most octahedral first row complexes.
Molecular Geometry | Boundless Chemistry A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. In the geometry, three atoms are in the same plane with bond angles of 120°; the other two atoms are on opposite ends of the molecule. Some elements in Group 15 of the periodic table form compounds of the type AX 5; examples include PCl 5 and AsF 5.
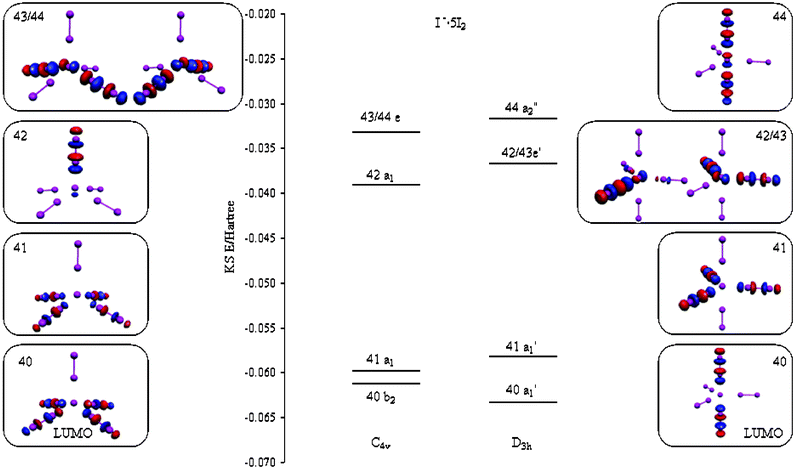
Trigonal BiPyramid Geometry - Elmhurst University The Lewis diagram is as follows: I = 7 e- x 3 = 21 e- -1 charge = 1 e- Total electrons = 22 e- With two atoms attached and three lone pair, the electron pair geometry is trigonal bipyramid. The molecular geometry is called linear. The triiodide ion is responsible for the blue-black color Iodine the element alone will not give the color.
a Trigonal bipyramidal coordination of Mo(3) and typical ... Download scientific diagram | a Trigonal bipyramidal coordination of Mo(3) and typical tetrahedral coordination of Mo; b examples of extensive distortion MoO4 polyhedra from tetrahedral geometry ...
Molecular Orbital Nanowires are promising building blocks for artificial photosynthesis · Nanocrystals can be designed with specific catalytic functionality
PDF MO Diagrams for More Complex Molecules MO Theory • MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection.
Construct a plausible Molecular Orbital (MO) energy diagram ... In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
(Color online) (a) MO 5 trigonal bipyramid and schematic ... Download scientific diagram | (Color online) (a) MO 5 trigonal bipyramid and schematic energy levels of the 3d bands of the TM ion in trigonal bipyramidal coordination, and (b) schematic energy ...





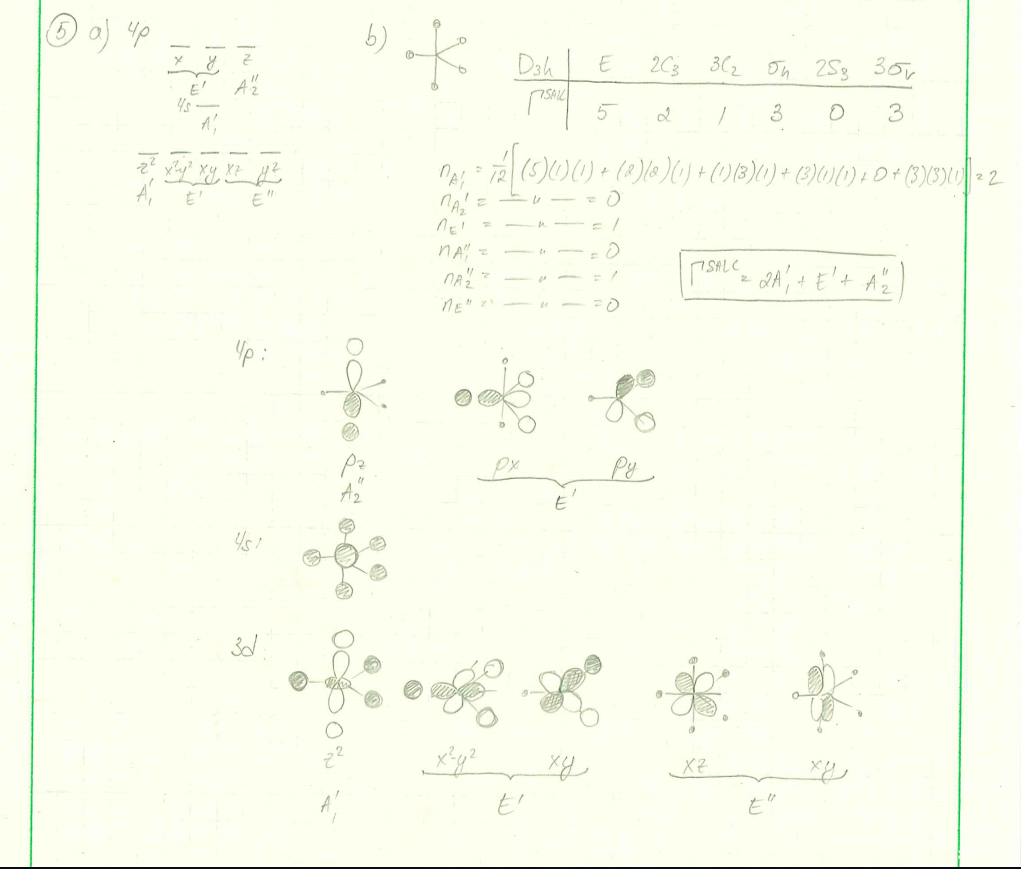








0 Response to "37 trigonal bipyramidal mo diagram"
Post a Comment