36 orbital diagram for sc
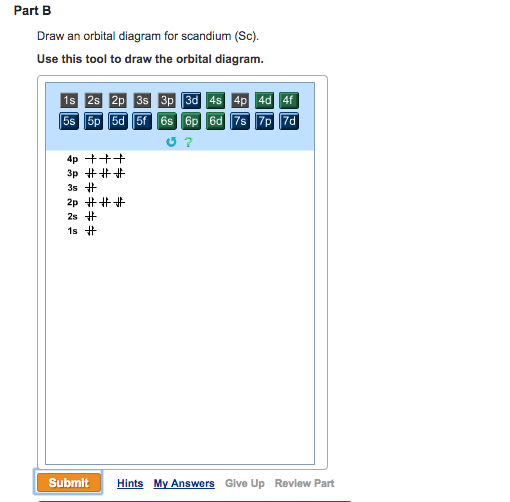
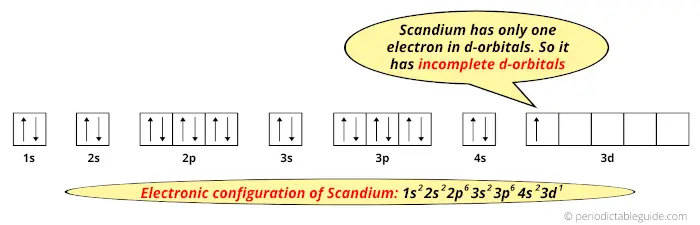
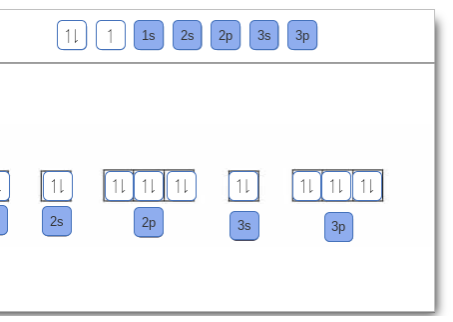
39 orbital diagram for scandium sc - Wiring Diagrams Manual Orbital diagram for scandium sc Scandium (Sc) electron configuration and orbital diagram Scientist Niels Bohr was the first to give an idea of the atom's orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. Draw An Orbital Diagram For Scandium (sc) Oct 10, 2018 · Answer to Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. Scandium has an atomic no. of Therefore electronic configuration of scandium (Sc) is: 1s2 2s2 2p6 3s2 3p6 3d1 4s2. Here, two electrons are in 4s orbital. For example, write the electron configuration of scandium, Sc: 1s2 2s2 2p6 3s2 3p6 4s2 3d1.
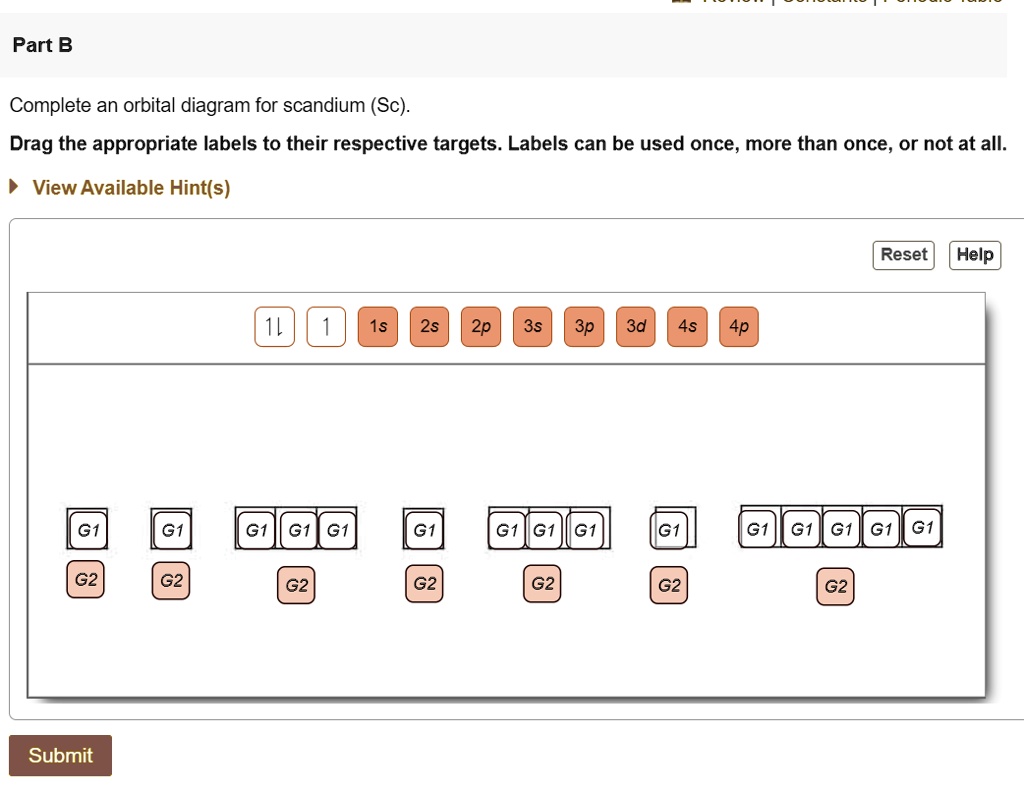
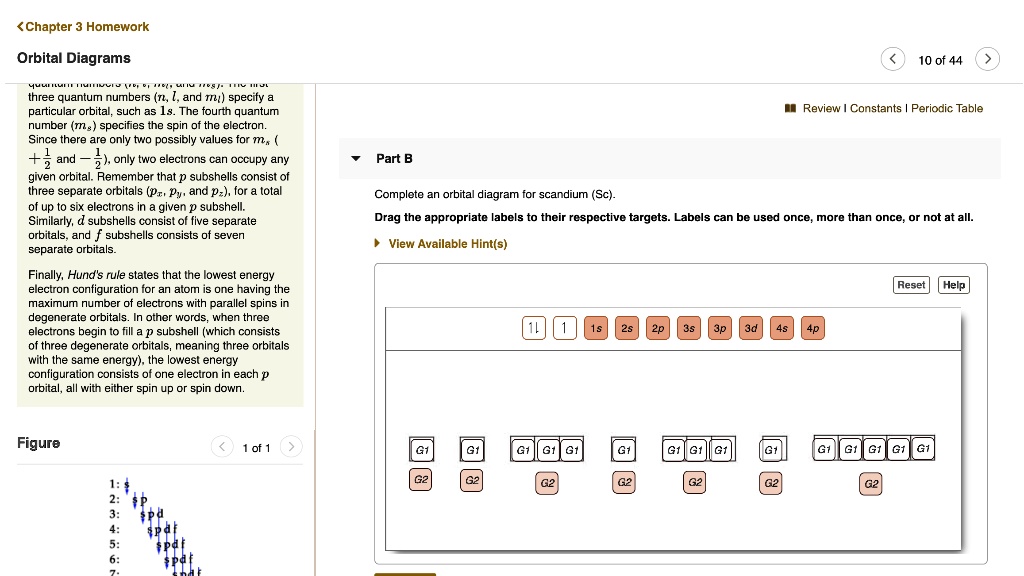
Complete An Orbital Diagram For Scandium (Sc). Complete an orbital diagram for Scandium (Sc). Drag the appropriate labels to their respective targets. Labels may be used once, more than once, or not at all. answer. atomic num.ba d scan/QYn a → scandi um half 21 elechon 3s 2s . Related. Categories Q&A. Leave a Comment Cancel reply.
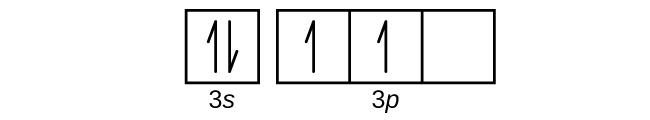
Orbital diagram for sc
Scandium(Sc) electron configuration and orbital diagram Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit(shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit] K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n2. For example, 1. n = 1 for K orbit. The electron holding capacity of K orbit is 2n2 = 2 × 12= 2 electrons. 2. For L orbit, n = 2. The electron holding capacity of the L orbit is 2n2 = 2 × 22= 8 electrons. 3. n=3 for M orbit. The maximum electron holding capacity in M orbit is 2n2 = 2 × 32 = 18 electrons. 4. n=4 for N orbit. The maximum electron holding capacity in N orbit is 2n2 = 2 × 42= 32 electrons. Therefore, the maximum electron holding capacity in the first shell... Orbital Diagram For Fe3+ Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms. PDF Electron configuration and orbital diagram for scandium For example, write the electron configuration of scandium, Sc: 1s2 2s2 2p6 3s2 3p6 4s2 3d1 . So for scandium the 1st and 2nd electron must be in 1s orbital, the 3rd and 4th in the 2s, the 5th through 10th in the 2p orbitals, etc. This is a memory device to remember the order of orbitals for the first two quantum numbers.
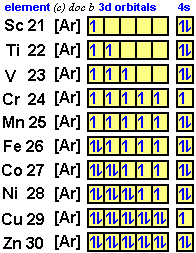
Orbital diagram for sc. How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. 38 orbital diagram for sc - Diagram For You An orbital diagram is similar What is the orbital diagram for. For example, write the electron configuration of sc andium, Sc : 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1. So for sc andium the 1 st and 2 nd electron must be in 1s orbital , the 3 rd and 4 th in the 2s, the 5 th through 10 th in the 2p orbital Solved Draw an orbital diagram for scandium (Sc). Use this ... Science; Chemistry; Chemistry questions and answers; Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. 1s, 2s, 2p, 3s, 3p, 3d, 4s ... Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30:
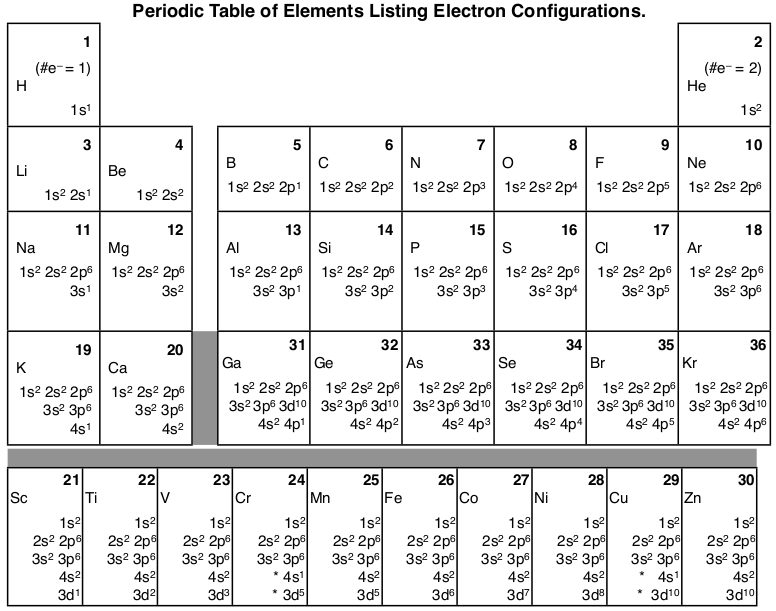
Chapter 8 Mastering Chemistry Flashcards - Quizlet Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Part C Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s2 2s2 2p1 is the electron ... Orbital diagrams and electron configurations Pre-AP (1 ... View Orbital diagrams and electron configurations Pre-AP (1).pptx from SC 101 at Miller Place High School. Orbital Diagrams, Electron Configurations, & Valence Electrons Bohr's Model: electrons Complete An Orbital Diagram For Scandium (sc). Answer to Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be us.Comprehensive information for the element Scandium - Sc is provided by this page including scores of properties, element names in many languages, most known nuclides and . Orbital Diagram For Scandium - 18 images - complete an ... solved draw an orbital diagram for scandium sc use thi. Orbital Diagram For Scandium. Here are a number of highest rated Orbital Diagram For Scandium pictures on internet. We identified it from honorable source. Its submitted by running in the best field. We give a positive response this kind of Orbital Diagram For Scandium graphic could ...
Electron configuration for Scandium (element 21). Orbital diagram Sc (Scandium) is an element with position number 21 in the periodic table. Located in the IV period. Melting point: 1539 ℃. Density: 2.99 g/cm 3 . Electronic configuration of the Scandium atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1. Electronic configuration of the Scandium atom in ascending order of the levels: Complete an orbital diagram for scandium (sc). Complete an orbital diagram for scandium (sc). - Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Draw an orbital diagram for scandium (Sc)? Jul 29, 2021 · Orbital Diagram For Scandium. draw orbital diagram scandium sc. Discover The Secrets Of Drawing Realistic Pencil Portraits. This will help you to achieve mastery in a very short period of time. All of these break down into 5 lessons of realistic facial features drawing. +) How To Draw A Realistic Eye. +) How To Draw A Realistic Nose. Solved Complete an orbital diagram for scandium (Sc). Drag ... Chemistry questions and answers. Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Question: Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used once, more than once ...
Draw An Orbital Diagram For Boron. Before we can draw a correlation diagram for B 2, we must first find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then, we rank them in order of increasing energy. Each boron atom has one 2s and three 2p valence orbitals. Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram.
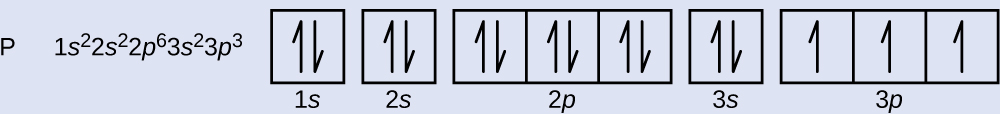
Chlorine(Cl) electron configuration and orbital diagram Orbital diagram for chlorine (Cl) Chlorine (Cl) excited state electron configuration Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of chlorine is 1s 2 2s 2 2p 6 3s 2 3p 5. The valency of the element is determined by electron configuration in the excited state.
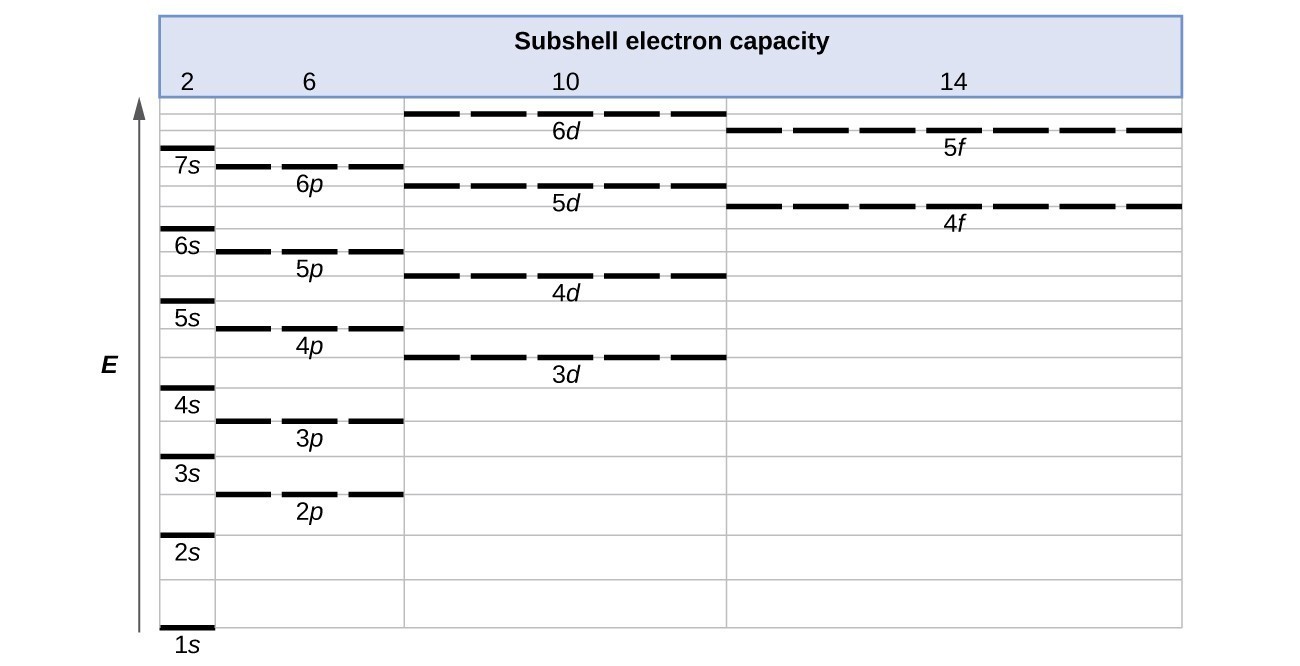
Complete An Orbital Diagram For Scandium (sc). Nov 09, 2018 · Answer to Part B Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used. Contrary to what you may have seen, for Sc and the remaining elements, the 4s is not lower in energy than the 3d. 21- Scandium electronic configuration with translucent orbitals
Orbital diagram for Sc? - Answers Definition of orbital diagram? An orbital diagram is used to show how the orbitals of a subshell areoccupied by electrons. The two spin projections are given by arrowspointing up (ms =+1/2) and...
Orbital Diagram For Scandium - Wiring Diagrams An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down. Scandium has an atomic no. of Therefore electronic configuration of scandium (Sc) is: 1s2 2s2 2p6 3s2 3p6 3d1 4s2. Here, two electrons are in 4s orbital. wiringall.com!
How to Write the Atomic Orbital Diagram for Scandium (Sc ... To write the orbital diagram for the Scandium atom (Sc) first we need to write the electron configuration for just Sc. To do that we need to find the number ...
What is the orbital diagram for Sc? - Study Hab What is the orbital diagram for Sc? Skip to content. Log in Create account Study Hab. Study Hab is a community of 2,991,959 amazing learners We're a place where learners ask for help for their tasks and share their knowledge. Create account Log in. Home Agriculture Anthropology ...
The phase diagram of the Sc-Zn system - ScienceDirect The phase diagram of the Sc-Zn system has been investigated using differential thermal analysis (DTA), metallographic analysis, X-ray diffraction (XRD) and electron microscopy. The Sc-rich side of the system (0 to 40 at.% Zn) has not been studied owing to the high reactivity and contamination of the samples by the container material (Mo).
chem 1201 HW Ch. 6 p 2 Flashcards | Quizlet Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. 1s: 2 arrows 2s: 2 arrows 2p: 2 arrows in all 3 orbitals 3s: 2 arrows 3p: 2 arrows in all 3 orbitals 4s: 2 arrows
PDF Electron configuration and orbital diagram for scandium For example, write the electron configuration of scandium, Sc: 1s2 2s2 2p6 3s2 3p6 4s2 3d1 . So for scandium the 1st and 2nd electron must be in 1s orbital, the 3rd and 4th in the 2s, the 5th through 10th in the 2p orbitals, etc. This is a memory device to remember the order of orbitals for the first two quantum numbers.
Orbital Diagram For Fe3+ Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms.
Scandium(Sc) electron configuration and orbital diagram Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit(shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit] K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n2. For example, 1. n = 1 for K orbit. The electron holding capacity of K orbit is 2n2 = 2 × 12= 2 electrons. 2. For L orbit, n = 2. The electron holding capacity of the L orbit is 2n2 = 2 × 22= 8 electrons. 3. n=3 for M orbit. The maximum electron holding capacity in M orbit is 2n2 = 2 × 32 = 18 electrons. 4. n=4 for N orbit. The maximum electron holding capacity in N orbit is 2n2 = 2 × 42= 32 electrons. Therefore, the maximum electron holding capacity in the first shell...





















0 Response to "36 orbital diagram for sc"
Post a Comment