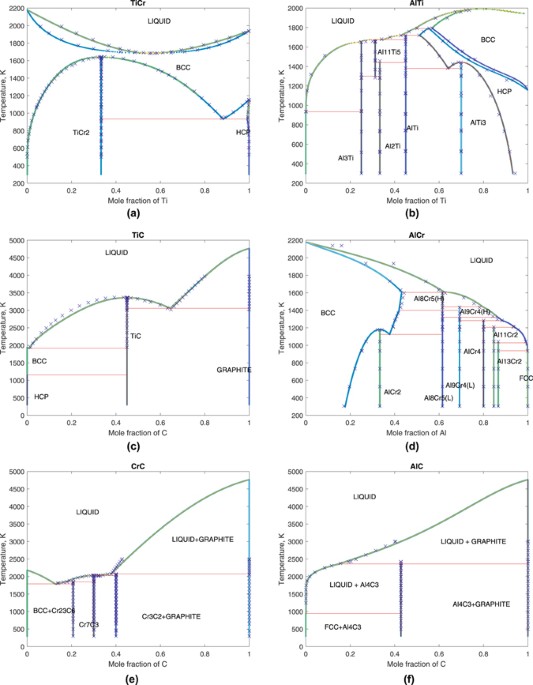
37 maximum solubility phase diagram
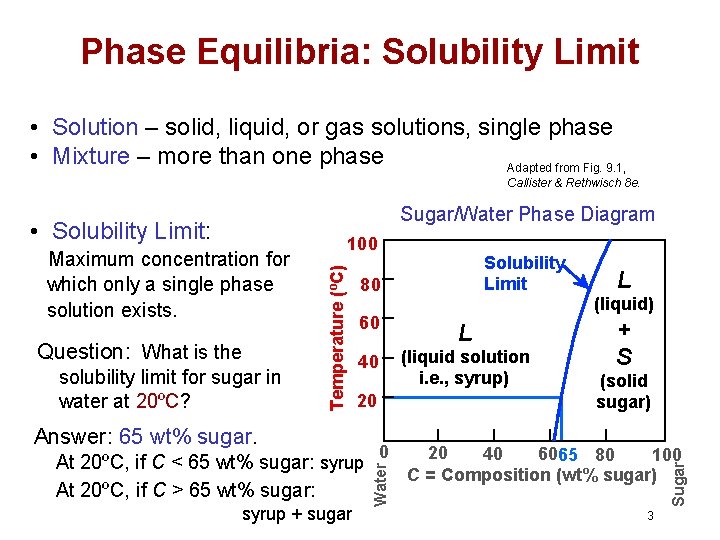
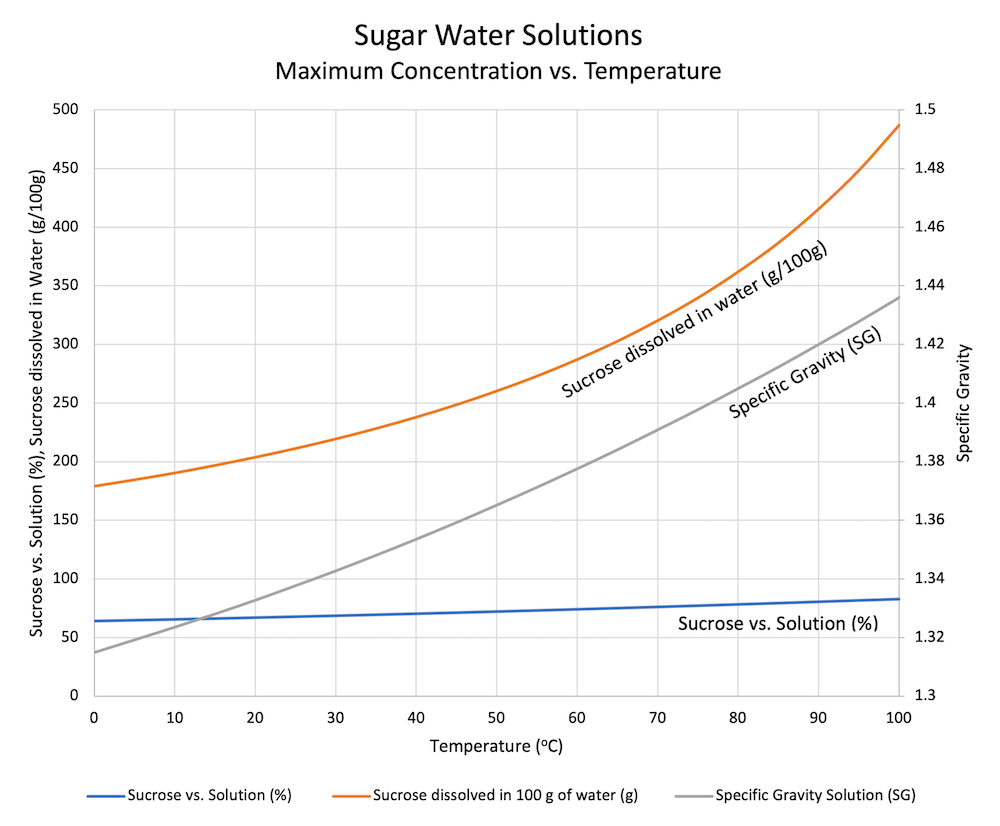
PDF Chapter 9: Phase Diagrams The Solubility Limit The Solubility Limit • Max. concentration for which only a solution occurs. -if Co < 65wt% sugar: syrup-if Co > 65wt% sugar: syrup + sugar • Solubility limit increases with T -e.g ... Phase Diagrams • Information about phases as function of T, Co, P • For this course: fractory.com › iron-carbon-phase-diagramIron-Carbon Phase Diagram Explained [with Graphs] - Fractory Mar 10, 2020 · This phase is a solid solution of carbon in FCC Fe with a maximum solubility of 2.14% C. On further heating, it converts into BCC δ-ferrite at 1395°C. γ-austenite is unstable at temperatures below eutectic temperature (727°C) unless cooled rapidly.
Iron Phase Diagram - Roy Mech The phased diagram includes four solid phases α Ferrite ..The solid solution of carbon in iron. At 0% C this is pure iron. BCC crystal structure. The maximum solubility of carbon in iron is 0,02% at 723oC. The carbon atoms are located in the crystal interstices. Austenite The solid solution of carbon in γ iron is called austenite .
Maximum solubility phase diagram
Phase diagram, solubility limit and hydrodynamic ... Phase diagram for cellulose in EmimAc-DMSO. Solid line corresponds to the maximal cellulose concentration soluble in EmimAc-DMSO calculated supposing no interactions between EmimAc and DMSO and 1 AGU binding NEmimAc = 2.5 mole of EmimAc; dashed lines correspond to AGU binding NEmimAc = 3 and 2 moles of EmimAc. [Solved] The maximum solubility of carbon in ferrite is- The maximum solubility of carbon in α - Iron is 0.025% at 723°. It is very soft and highly magnetic. It is in-fact softest structure that appears in Iron - Carbon equilibrium diagram. Pearlite: It is formed by the decomposition of austenite at 723°C. Phase Diagram: Meaning and Types | Material Engineering Hardness of pure material is very poor and maximum strength will be at the point of maximum solid solubility. Influence of Alloying Elements on Phase Diagram: Alloys elements addition always reduces eutectoid composition i.e.% of C while they may increase or decrease the eutectoid temperature.
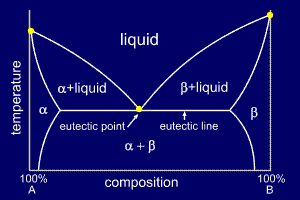
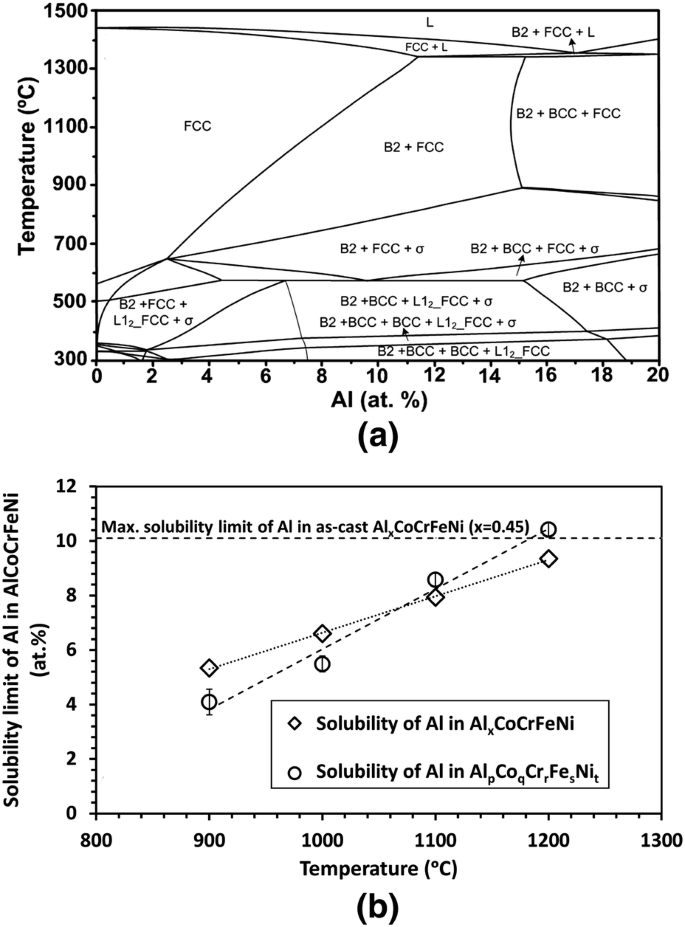
Maximum solubility phase diagram. Alloys - limited solubility of components in solid state ... The solvus lines for the respective components are shown in the phase diagram in purple and green. They therefore reflect the maximum solubility of the stored component. As the temperature drops, the solubility of the components in the other lattice decreases! Solid Solubility - an overview | ScienceDirect Topics 2.6 Structural analysis of Mg-RE alloys containing Gd. The maximum solid solubility of Gd in Mg is 23.5 wt.%. When added to Mg, Gd forms a Mg 5 Gd phase with a high melting point of 548 °C. Figure 2.1024 shows the as-cast microstructure (a) and the solution-treated micro-structure (b) of the Mg-14Gd binary alloy. What is Alpha in phase diagram? - JanetPanic.com Solubility Limit of a component in a phase is the maximum amount of the component that can be dissolved in it (e.g. alcohol has unlimited solubility in water, sugar has a limited solubility, oil is insoluble). What is the binary Al-Si phase diagram? The binary Al-Si phase diagram was initially studied by Fraenkel of Germany in 1908. At 700C (1290F), what is the maximum solubility (a) of Cu ... In this exercise we have to calculate the maximum of each component in the solution:. a) 4%. b) 5%. We need a Cu-Ag phase diagram to answer these questions (see diagram). Yours may differ slightly from the one I used. (a) Cu in Ag. The horizontal red line at 700 °C cuts the right-hand solvus at about 4 % Ag-96 % Cu.
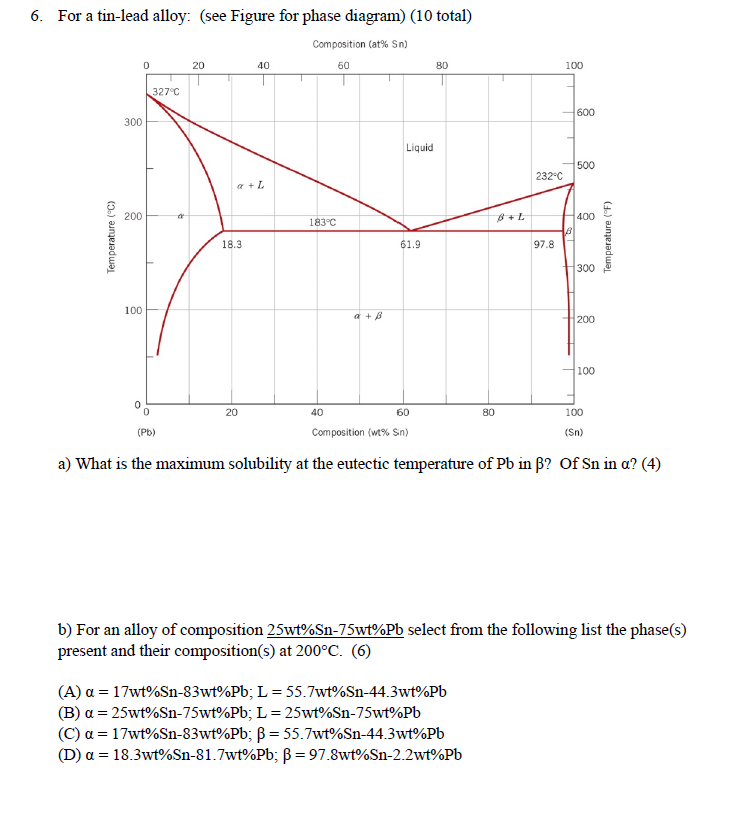
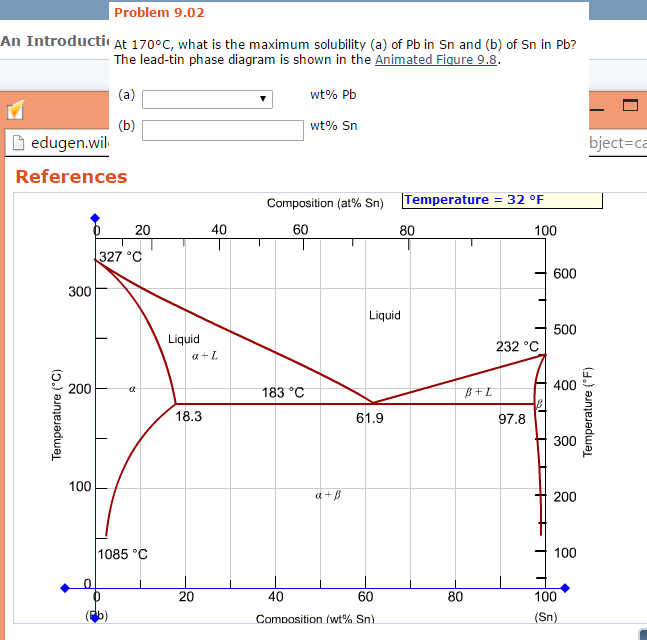
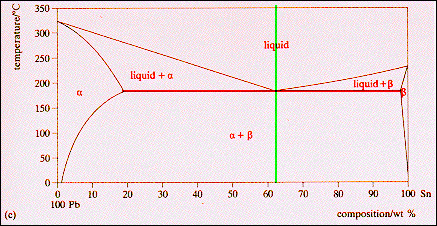
› metallurgy › metalsSolid Solution of Metals: With Diagram | Metallurgy It the atomic diameters differ by more than 15 percent, the size factor is unfavorable and the solid solubility is small. Metals like silver and gold have a difference of 0.2%; nickel and copper of 2.7%, and show complete solid solubility. But zinc and copper have 4.2% difference with maximum solubility of 38.4 wt.% Zn. materials - Princeton University The maximum solid solubility of tin in lead occurs at the eutectic temperature (183 C) and the a-phase at this temperature has the composition Pb-19.2 wt % Sn. Similarly, at the tin rich end of the composition range, lead has its maximum solid solubility in tin at the eutectic temperature and the b -phase at this temperature has the composition ... Solubility Limits and Determining Phase Present ... For 60+ videos on Engineering Materials › metallurgy › ironIron-Carbon Equilibrium Diagram | Metallurgy It is an interstitial solid solution of carbon in delta iron having BCC structure. It has maximum solubility of carbon of 0.09% at 1495°C. It is a high temperature phase and is a high temperature manifestation of a-ferrite. (iv) Cementite, Iron Carbide, Fe 3 C: It is an interstitial intermediate compound having a fixed carbon content of 6.67%.
marinerspoint.in › iron-carbon-equilibrium-diagramIron Carbon Equilibrium Diagram with Explanation [Phase ... Nov 28, 2021 · This phase is a solid solution of carbon in FCC Fe with a maximum solubility of 2.14% C. On further heating, it converts into BCC ferrite at 1395°C. γ-austenite is unstable at temperatures below eutectic temperature (727°C) unless cooled rapidly. Phase Diagram: Meaning and Types | Material Engineering Hardness of pure material is very poor and maximum strength will be at the point of maximum solid solubility. Influence of Alloying Elements on Phase Diagram: Alloys elements addition always reduces eutectoid composition i.e.% of C while they may increase or decrease the eutectoid temperature. [Solved] The maximum solubility of carbon in ferrite is- The maximum solubility of carbon in α - Iron is 0.025% at 723°. It is very soft and highly magnetic. It is in-fact softest structure that appears in Iron - Carbon equilibrium diagram. Pearlite: It is formed by the decomposition of austenite at 723°C. Phase diagram, solubility limit and hydrodynamic ... Phase diagram for cellulose in EmimAc-DMSO. Solid line corresponds to the maximal cellulose concentration soluble in EmimAc-DMSO calculated supposing no interactions between EmimAc and DMSO and 1 AGU binding NEmimAc = 2.5 mole of EmimAc; dashed lines correspond to AGU binding NEmimAc = 3 and 2 moles of EmimAc.



![Solved] The maximum solubility of carbon in ferrite is-](https://storage.googleapis.com/tb-img/production/19/08/F3_S.S_M.P_17.08.19_D%201.png)













0 Response to "37 maximum solubility phase diagram"
Post a Comment