39 energy diagram with catalyst
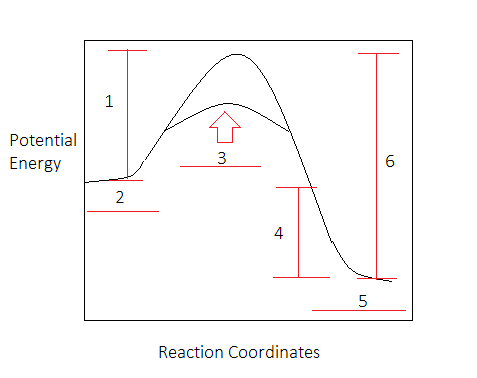
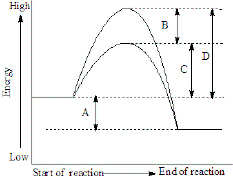
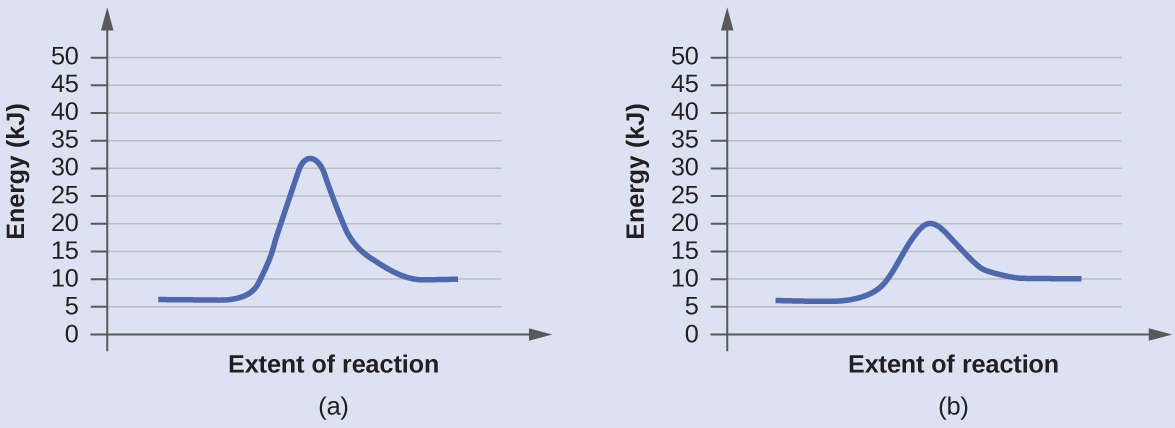
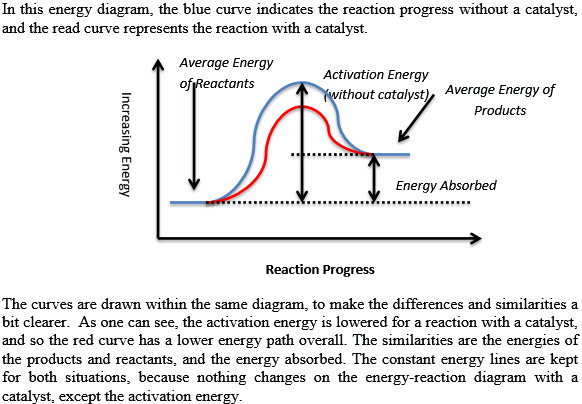
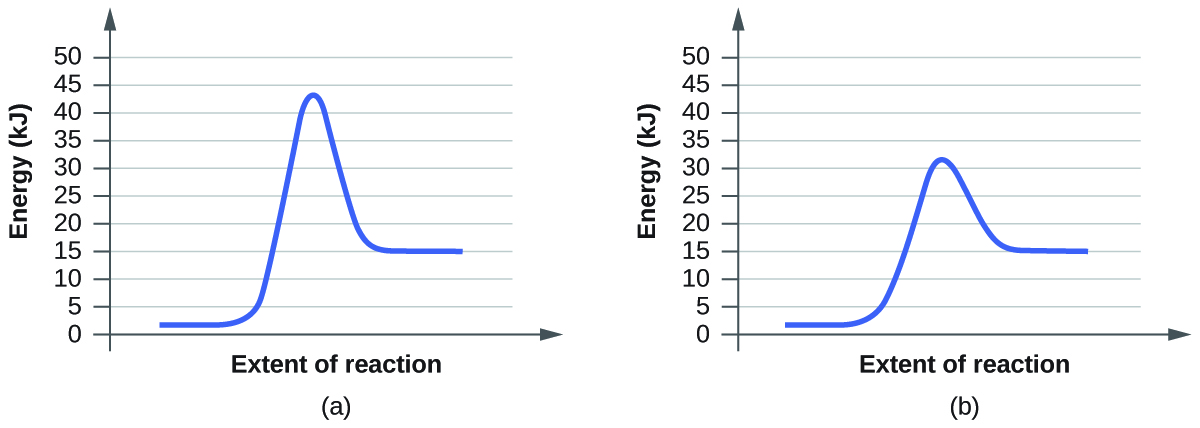
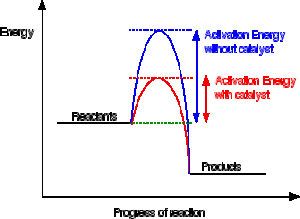
12.7 Catalysis - Chemistry - opentextbc.ca A catalyst does not affect the energy of reactant or product, so those aspects of the diagrams can be ignored; they are, as we would expect, identical in that respect. There is, however, a noticeable difference in the transition state, which is distinctly lower in diagram (b) than it is in (a). Explain with the help of a potential energy diagram that ... Potential energy barriers for catalyzed and uncatalyzed reactions; The potential energy diagram compares the potential energy barriers for the catalysed and uncatalysed reactions. The barrier for uncatalysed reaction (E a) is larger than that for the same reaction in the presence of a catalyst E a.
Energy Profiles (Energy Diagrams) Chemistry Tutorial The catalyst provides an alternative, lower-energy, pathway for the reaction to follow, using a lower-energy intermediate product (lower-energy activated complex). If the catalyst is a solid, it can do this by providing a surface on which the reactant molecules can "stick" in the correct orientation, increasing the rate at which successful ...

Energy diagram with catalyst
catalysis - Potential Energy Diagrams and the Effect of ... Why does a potential energy diagram showing the effect of a catalyst on activation energy not move left on the reaction pathway scale (compared to uncatalysed reaction) if a catalyst speeds up reac... › van-krevelen-diagramVan Krevelen Diagram - an overview | ScienceDirect Topics The van Krevelen diagram was used to characterize the chemical composition of wine, allowing its authentication [83]. Specific regions of the van Krevelen diagram are associated with specific classes of compounds, such as carbohydrates (for H/C 2 and O/C 1), or lipids (for O/C < 0.2 and H/C 2) [84]. Potential Energy Diagram of Catalyzed and Uncatalyzed ... Analyzing the potential energy diagram of a regular/uncatalyzed and a catalyzed (adding a catalyst) reaction. Remember that the 🔼H of reaction remains the s...
Energy diagram with catalyst. Energy Diagrams, Catalysts, and Reaction Mechanisms It's time to learn a little more about a chemical reaction. How do molecules have to be arranged and how much energy do they have to collide with? What's a c... Energy Diagram Catalyzed Vs Uncatalyzed Reaction Catalysis is the process of increasing the rate of a chemical reaction by adding a substance Catalyzed reactions have a lower activation energy (rate-limiting free energy of activation) than the corresponding uncatalyzed reaction, resulting in a higher reaction . This effect can be illustrated with an energy profile diagram. eere-exchange.energy.gov › defaultFinancial Opportunities: Funding Opportunity Exchange - Energy This funding opportunity announcement (FOA) is being issued by the U.S. Department of Energy’s (DOE) Office of Energy Efficiency and Renewable Energy (EERE) Solar Energy Technologies Office (SETO) to invest in innovative research that accelerates the large-scale development and deployment of solar technology to support an equitable transition to a decarbonized electricity system by 2035 and ... › questions › 18836631. Use the table to answer the question: Bond Bond Energy H–H ... Feb 03, 2022 · B. the kinetic energy of the reactants of a system after the reaction has begun C. the total potential energy of the bonds of the reactants in a system D. the energy released when atomic bonds are broken 10. Manganese dioxide acts as a catalyst in the decomposition of H2O2 into water and oxygen gas. Which statement best describes this function ...
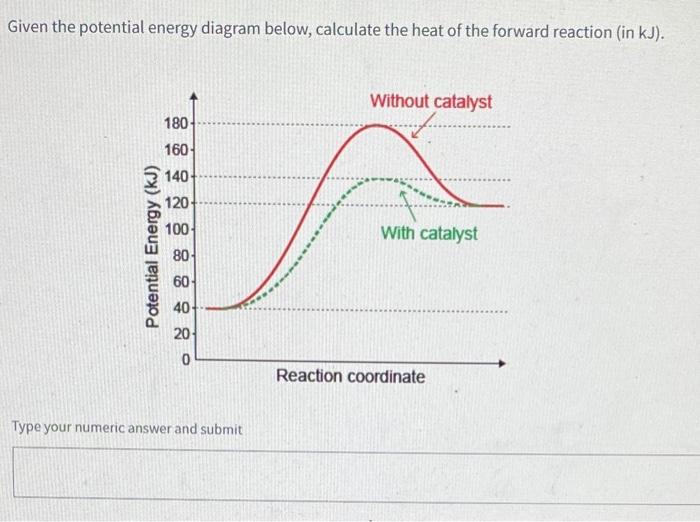
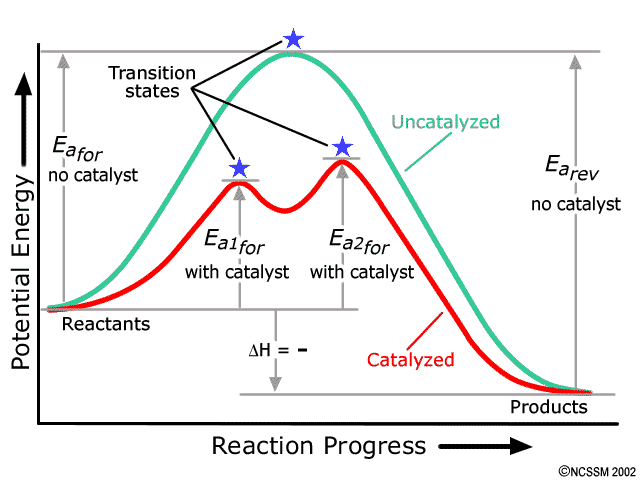
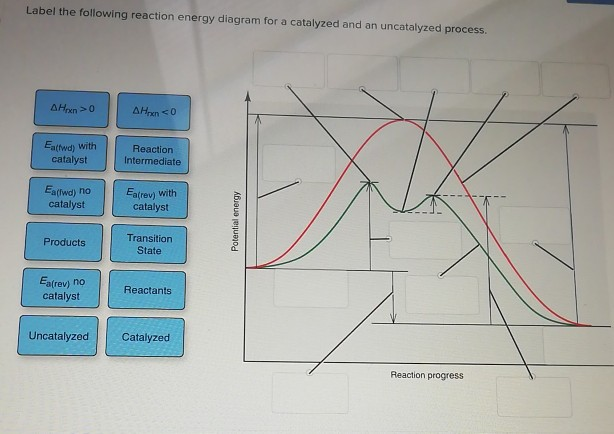
Drawing the Reaction Energy Diagram of a Catalyzed ... For the following simplified equation, label the reaction energy diagram with and without a catalyst. {eq}X + Y \rightarrow Z {/eq} Step 1: Determine the energies of the reactants and the products Catalysts potential energy diagram - Big Chemical Encyclopedia Catalysts potential energy diagram To see how the catalyst accelerates the reaction, we need to look at the potential energy diagram in Fig. 1.2, which compares the non-catalytic and the catalytic reaction.For the non-catalytic reaction, the figure is simply the familiar way to visualize the Arrhenius equation the reaction proceeds when A and B collide with sufficient energy to overcome the ... Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... Enthalpy, Energy Diagrams, and Catalysts - YouTube Here is a bit of explanations about reaction enthalpies, energy diagrams for endothermic and exothermic reactions and how catalysts affect energy diagrams
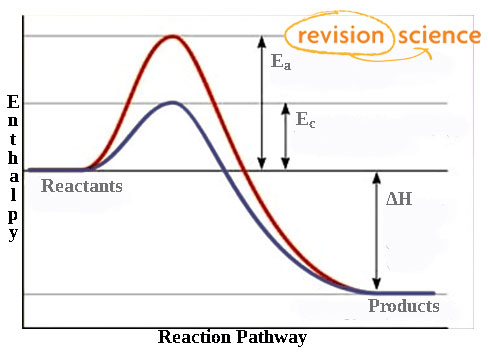
› articles › s41467/018/03795-8Heterogeneous Fe3 single-cluster catalyst for ammonia ... Apr 23, 2018 · For Fe C7 site, although the transition state energy of *N 2 dissociation is only −0.10 eV with respect to the gas phase N 2 and clean surface, the adsorption energy of two *N is as high as −2 ... Energy Diagram Catalyzed Vs Uncatalyzed Reaction Label the energy diagram and answer the question that follows% (1). The decomposition of hydrogen peroxide is exothermic. The reaction is catalyzed by iodide ion. The equation for the uncatalyzed reaction is. 2 H 2 O 2 (l) 2 H 2 O (l) + O 2 (g) Sketch a possible graph for this reaction, first without a catalyst and then with a catalyst. What does a catalyst do to the energy diagram? | Popular ... Energy diagrams are useful to illustrate the effect of a catalyst on reaction rates. Catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy diagram in Figure 7.14), and therefore increase the reaction rate. Potential Energy Diagram - Catalyst Added - YouTube Sketch the potential energy diagram for an exothermic reaction with a catalyst added.
phet.colorado.edu › en › simulationsReactions & Rates - Reaction | Kinematics | Concentration ... Explore what makes a reaction happen by colliding atoms and molecules. Design experiments with different reactions, concentrations, and temperatures. When are reactions reversible? What affects the rate of a reaction?
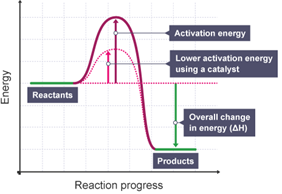
PPT Activation Energy and Catalyst Reaction Coordinate Diagrams It is helpful to visualize energy changes throughout a process on a reaction coordinate diagram like this one for the rearrangement of methyl isonitrile. Reaction Coordinate Diagrams The diagram shows the energy of the reactants and products (and, therefore, E). The high point on the diagram is the transition state.
Energy Changes in Catalytic Reactions - QS Study Energy Changes in Catalytic Reactions . The question may arise as to how does the catalyst enhance the rate of reactions? This can be explained with the help of an energy diagram as in Figure. It has been shown that a catalyzed reaction has lower activation energy than the same reaction taking place in the absence of the catalyst. This is ...
en.wikipedia.org › wiki › Phase_(matter)Phase (matter) - Wikipedia The energy required to induce the phase transition is taken from the internal thermal energy of the water, which cools the liquid to a lower temperature; hence evaporation is useful for cooling. See Enthalpy of vaporization. The reverse process, condensation, releases heat.
Potential energy diagram catalyst effect - Big Chemical ... Figure 16-15 Potential energy diagrams showing the effect of a catalyst. The catalyst provides a different mechanism, corresponding to a lower-energy pathway, for the formation of the products. A catalyzed reaction typically occurs in several steps, each with its own barrier, but the overall energy barrier for the net reaction,, is lower than ...
How do catalysts affect energy diagrams ... Energy diagrams are useful to illustrate the effect of a catalyst on reaction rates. Catalysts decrease the activation energy required for a reaction to proceed (shown by the smaller magnitude of the activation energy on the energy diagram in Figure 7.14), and therefore increase the reaction rate.
Catalysis - Chemistry - University of Hawaiʻi A catalyst does not affect the energy of reactant or product, so those aspects of the diagrams can be ignored; they are, as we would expect, identical in that respect. There is, however, a noticeable difference in the transition state, which is distinctly lower in diagram (b) than it is in (a).
Potential Energy Diagram of Catalyzed and Uncatalyzed ... Analyzing the potential energy diagram of a regular/uncatalyzed and a catalyzed (adding a catalyst) reaction. Remember that the 🔼H of reaction remains the s...
› van-krevelen-diagramVan Krevelen Diagram - an overview | ScienceDirect Topics The van Krevelen diagram was used to characterize the chemical composition of wine, allowing its authentication [83]. Specific regions of the van Krevelen diagram are associated with specific classes of compounds, such as carbohydrates (for H/C 2 and O/C 1), or lipids (for O/C < 0.2 and H/C 2) [84].
catalysis - Potential Energy Diagrams and the Effect of ... Why does a potential energy diagram showing the effect of a catalyst on activation energy not move left on the reaction pathway scale (compared to uncatalysed reaction) if a catalyst speeds up reac...


/catalystenergydiagram-56a12b265f9b58b7d0bcb2fe.jpg)

















0 Response to "39 energy diagram with catalyst"
Post a Comment