36 cs molecular orbital diagram
Chem 59-250 Symmetry of orbitals and functions yz y, R x 1-1-1 1 B 2 xz xy x 2,y 2,z 2 x, R y R z z-1 1-1 1 B 1-1-1 1 1 A 2 1 1 1 1 A 1 σ ’ v (yz) σ v (xz) C 2 E C 2V z y x z y x A p z orbital has the same symmetry as an arrow pointing along the z-axis. November 13, 2015 - Linear. The best place to start when trying to figure out a molecule's geometry is its Lewis structure. Carbon disulfide, "CS"_2, will have a total of 16 valence electrons, 4 from the carbon atom and 6 from each of the two sulfur atoms. The central carbon atom will form double bonds with the ...
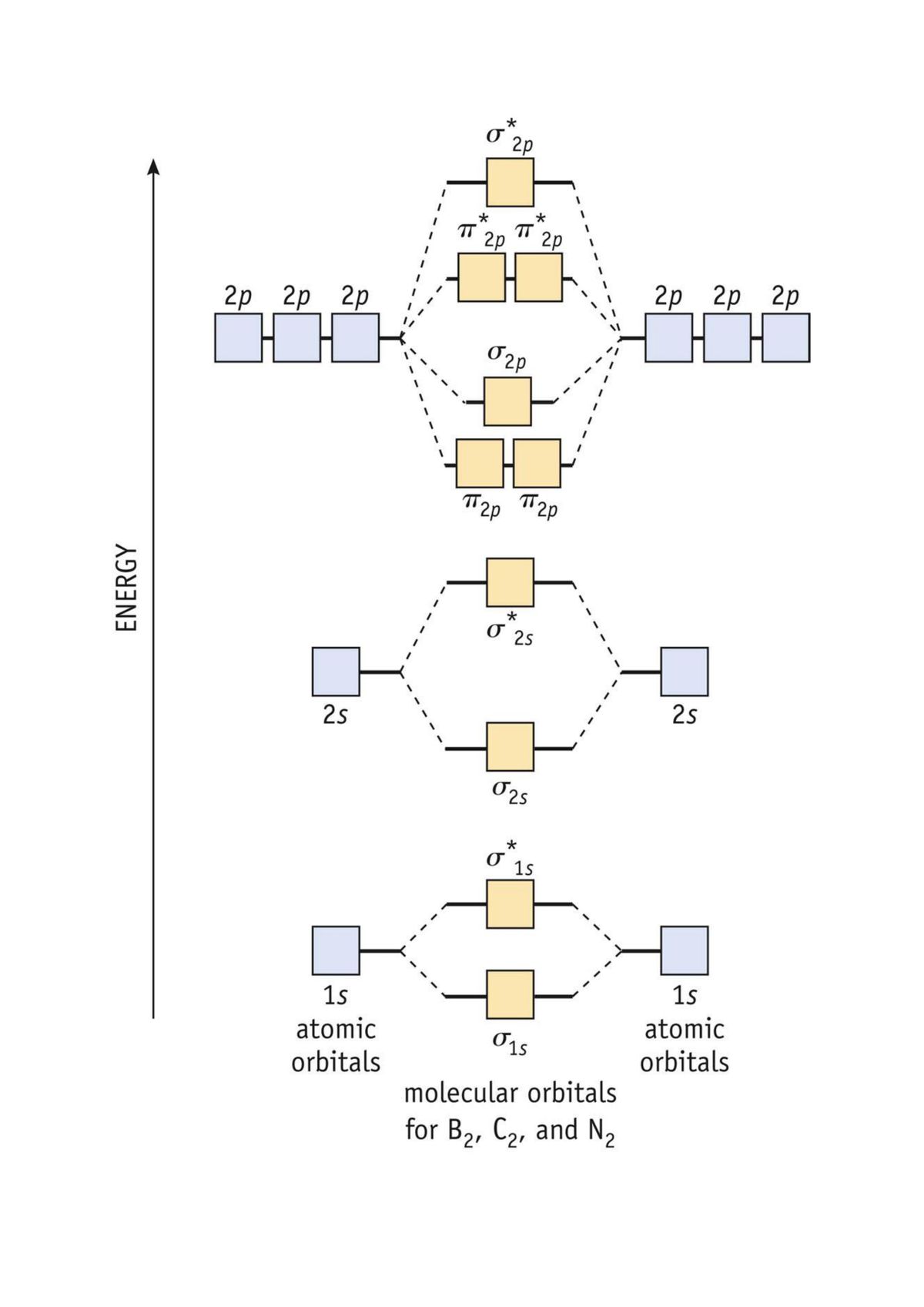
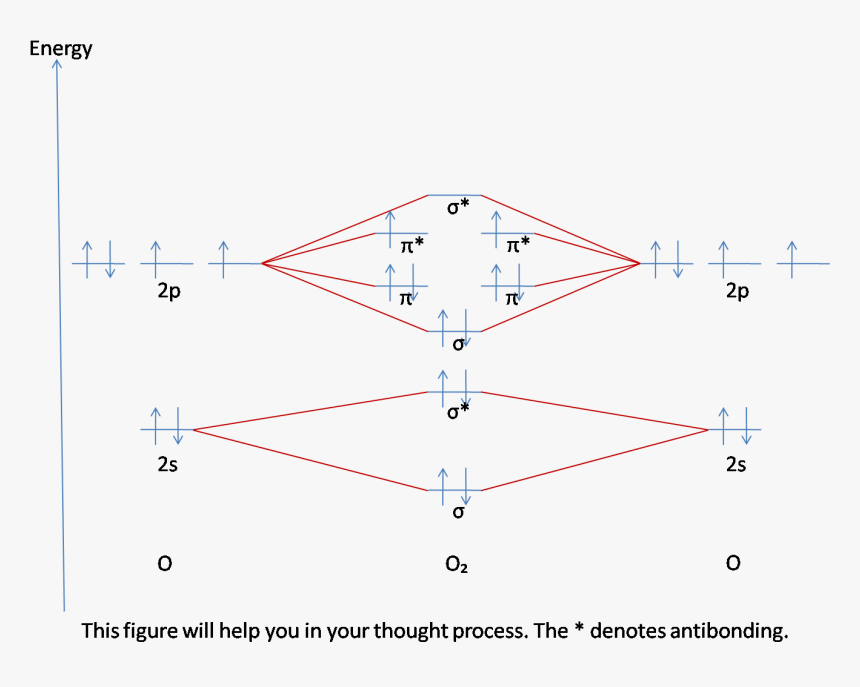
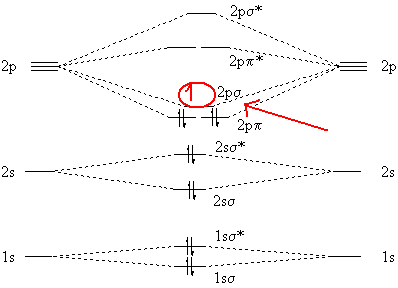
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Cs molecular orbital diagram
Answer to Draw the MO diagram for CS2 . Showing the possible orbitals for each atom or group. Which would be bonding, non-bonding ... Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we’ll see that symmetry will help us treat larger molecules in Download scientific diagram | Graphical representation of the LUMO orbitals for CO 2 , OCS and CS 2 calcu- from publication: Kinetics of the Reactions of Water, Hydroxide Ion and Sulfide Species ...
Cs molecular orbital diagram. The molecular structure has been optimized at the B3LYP/6-31g* level of theory. Charges used for electrostatic maps are computed using the NBO method. The molecular vibrations are January 4, 2021 - A molecular orbital diagram that can be applied to any homonuclear diatomic molecule with two identical alkali metal atoms (Li2 and Cs2, for example) is shown in part (a) in Figure \(\PageIndex{4}\), where M represents the metal atom. Only two energy levels are important for describing the ... Molecular orbitals and bond lengths found with Spartan can be used to better understand how the actual structure of a molecule or ion relates to its resonance structures. In this experiment, the valence atomic orbitals for hydrogen, carbon, nitrogen, and sulfur atoms will be calculated. Molecular orbitals for H 2, N 2, CS, molecular orbital energy level diagram for CS below by: i. Sketching and naming . only : the molecular orbitals (MOs). ii. ... Label all atomic and molecular orbitals on your diagram, and include tie lines to show the linear combinations that form each molecular orbital.
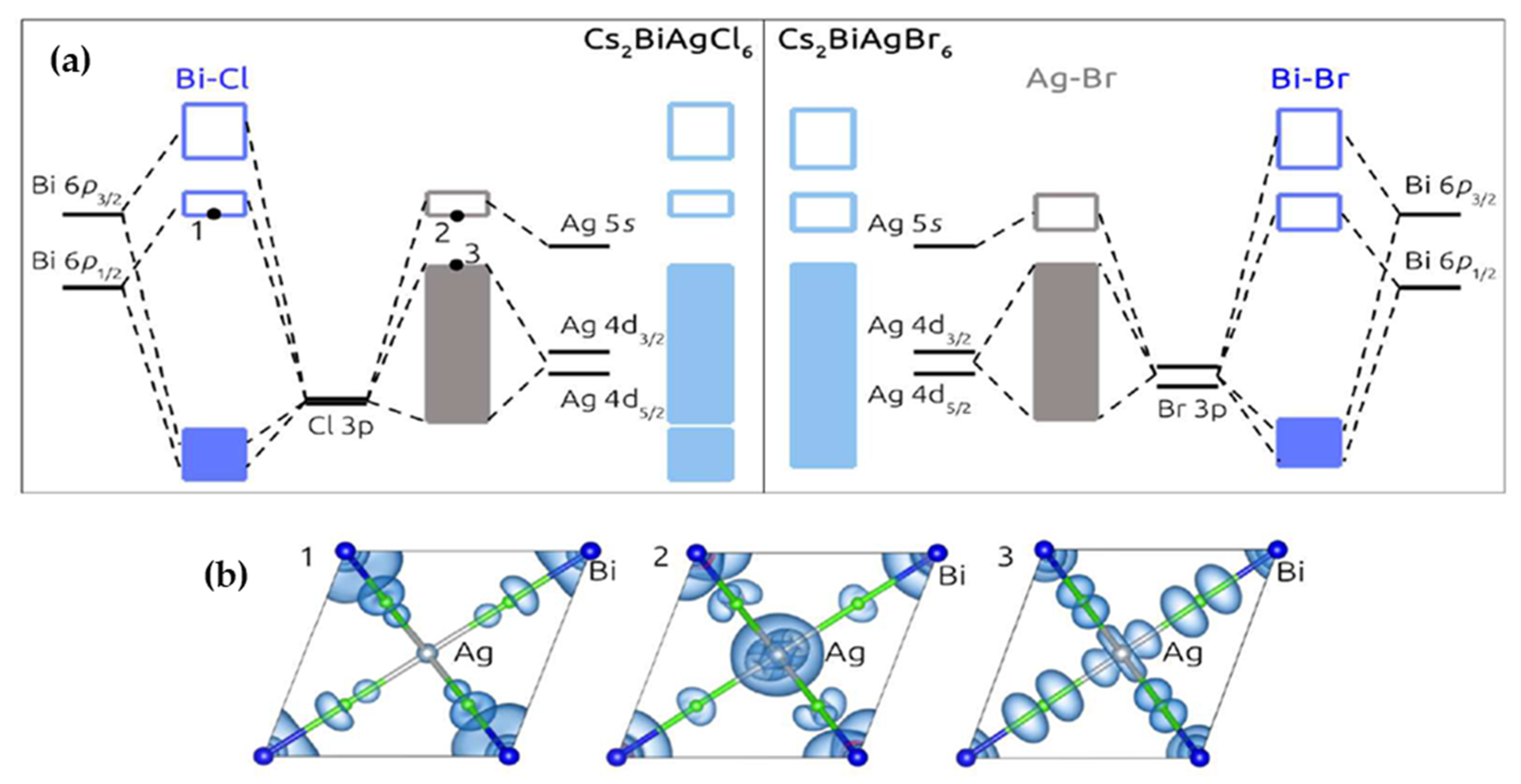
Molecular orbitals provide a great model for demonstrating molecule bonding via molecular orbital theory. Types of Molecular Orbitals. According to molecular orbital theory, some types of molecular orbitals are formed by the linear combination of atomic orbitals. These orbitals are described in more detail below. [Cs(18-crown-6) 2] + e ... Home / Structure and Bonding / Atomic Orbitals / Molecular orbitals in Carbon Monoxide. Molecular orbitals in Carbon Monoxide. CONTROLS > Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Diatomic Species by Molecular Orbital Theory. Even rather simple molecular orbital (MO) theory can be used to predict which homonuclear diatomic species - H 2, N 2, O 2, etc. - will exist, explain many properties - for example why O 2 is a paramagnetic diradical - and identify the important frontier molecular orbitals (FMOs). November 23, 2015 - 12/20: Andy's paper on supported nanocrystal catalysts is published in Chem. Mater.! 10/20: Our joint paper with the Klimov group using ALD to produce CMOS circuit elements from CIS quantum dot films is published in Nature Comms. 9/20: Our paper with Adam Moule's group on electron tomography ...
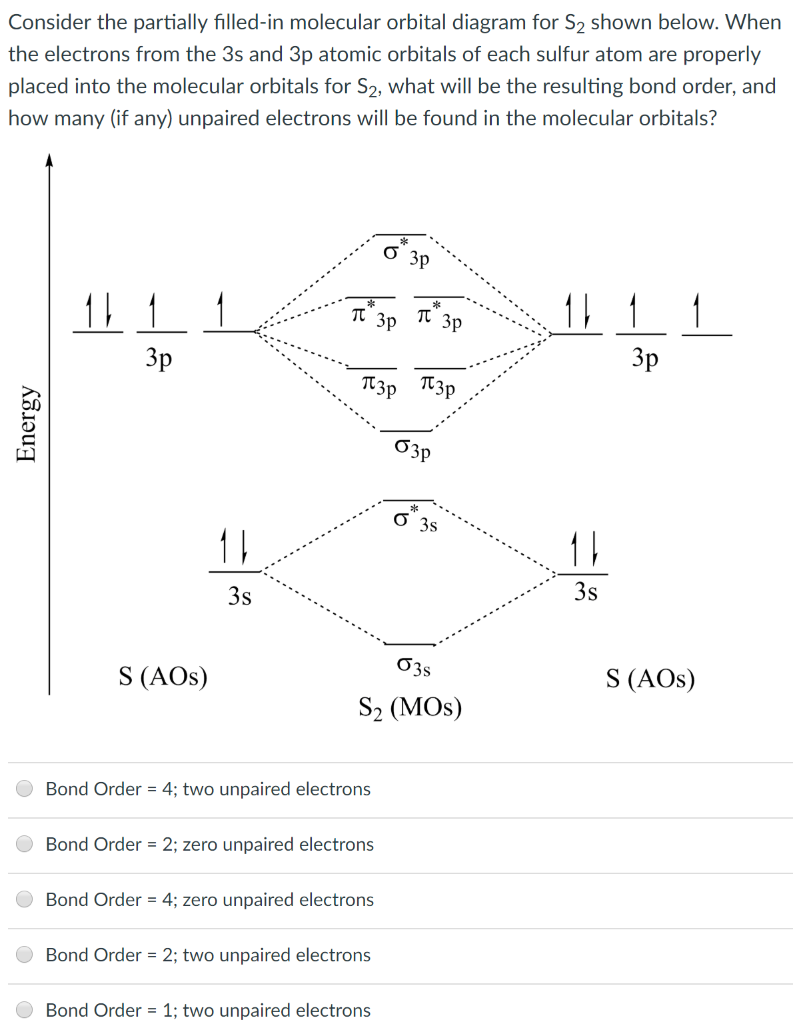
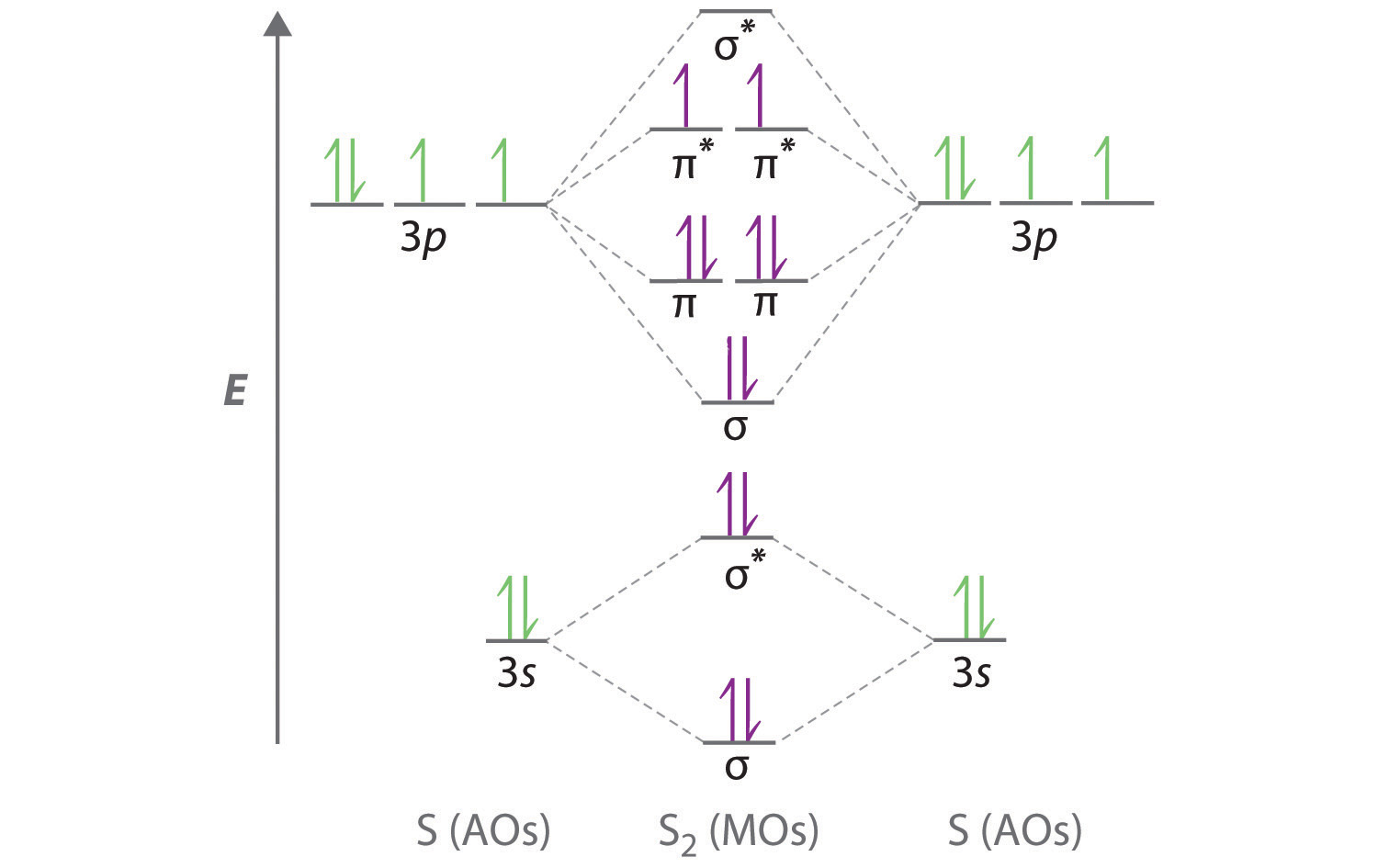
For the elements Cs, F, and Cl, the order of increasing electronegativity is. ... Use the orbital diagram shown to determine which of the following is most stable. O2^2+ Use the molecular orbital diagram show to determine which of the following are paramagnetic. F2^2+ How many of the following molecules possess dipole moments? Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals. A quick explanation of the molecular geometry of CS2 including a description of the CS2 bond angles. Looking at the CS2 Lewis structure we can see that there... Draw a molecular orbital energy diagram for CS (carbon sulfide). Compare the outcome with CO, and predict the coordination chemistry of CS.
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
4 answersgot a question telling us to produce an M O diagram. A molecular little diagram for F two plus and status box. So first things first.
FREE Answer to 10. Use the molecular orbital diagram to determine the configuration for Cs. In this order, what are the bond order...
Molecular orbital diagrams provide qualitative information about the structure and stability of the electrons in a molecule. This article explains how to create molecular orbital diagrams in L a T e X by means of the package MOdiagram.For information about the more traditional molecular structure diagrams see our documentation about chemistry formulae.
The molecular orbital energy level diagram of oxygen molecule is given as follows : Bond order 2 N b − N a = 2 8 − 4 = 2 Thus, oxygen molecule has two bonds. i.e., one is bond and one p bond.
Study at one of Australia’s leading science faculties in tertiary education. Discover our course offerings as well as our impactful research and collaborations.
August 16, 2008 - Export articles to Mendeley · Get article recommendations from ACS based on references in your Mendeley library
CS2 Lewis Structure, Hybridization, Polarity and Molecular Shape. CS2 is an abbreviated form of Carbon Disulphide. This molecule has two Sulphur atoms and one Carbon atom. To understand the hybridization, molecular geometry and the polarity of this molecule it is essential to under its Lewis structure. ぷっくりお花のキーホルダー ...
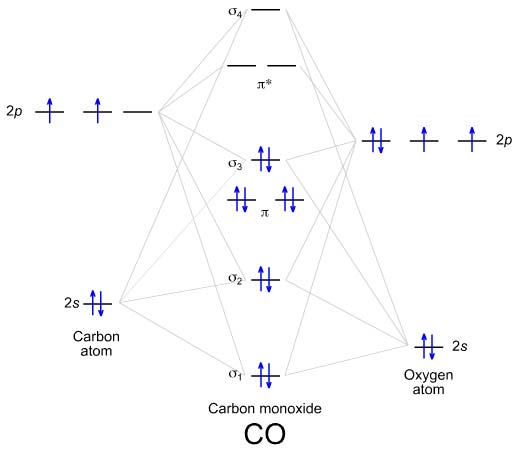
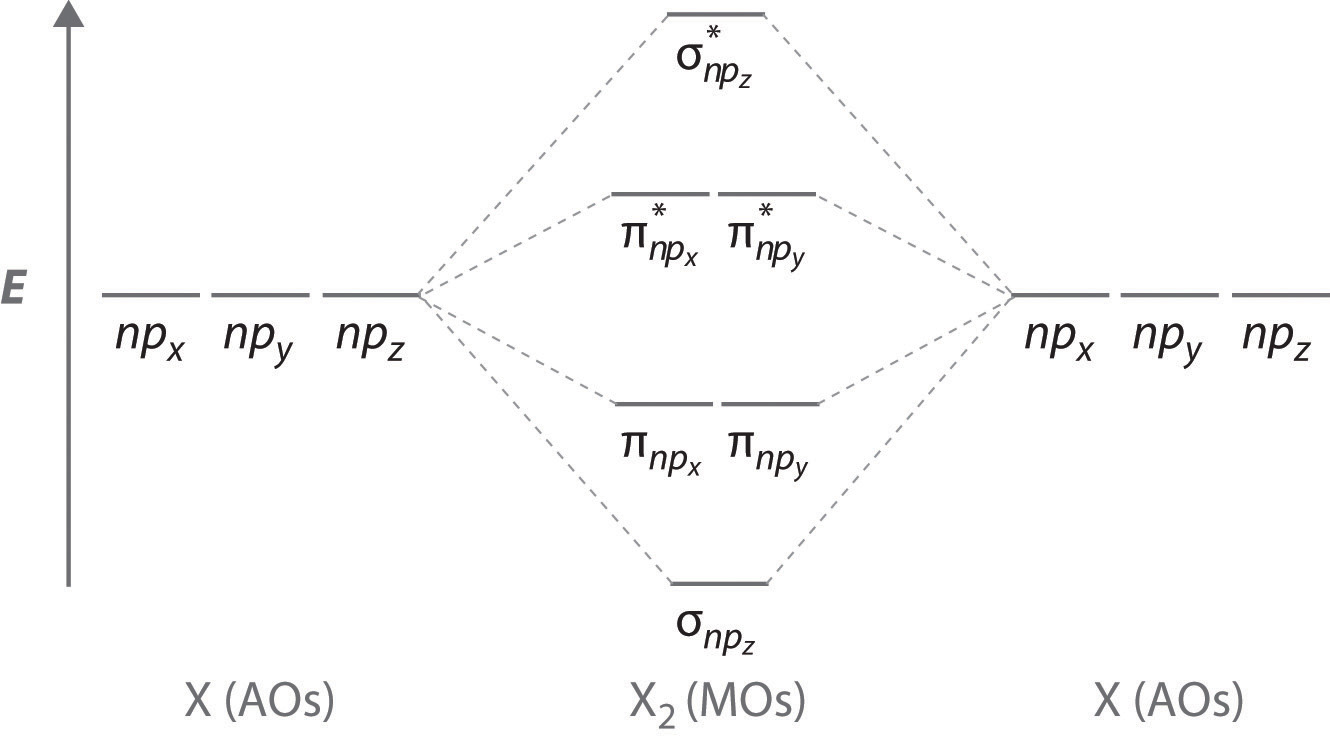
These must make 4 sigma symmetry molecular orbitals with an average energy equal to the average energy of the 4 atomic orbitals. In N 2 and in most other diatomic molecules (NO, NS, CO, CS) there are 4 sigma symmetry molecular orbitals made from a mixing of the 2s and 2p z atomic orbitals on each atom.
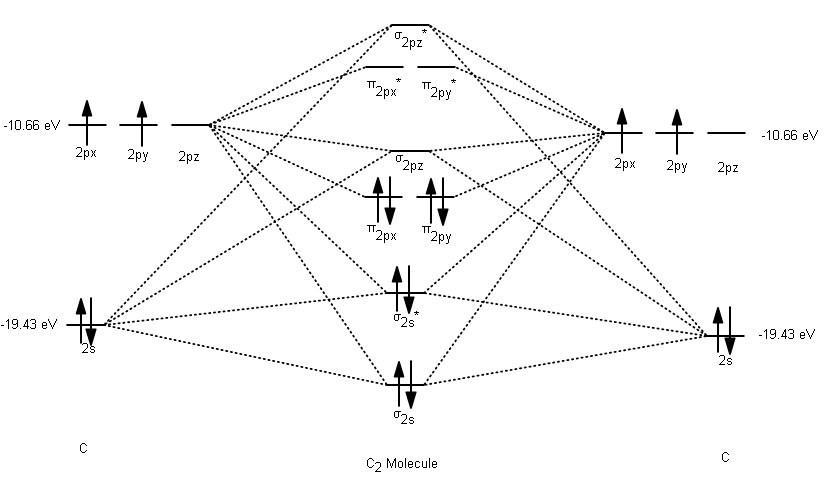
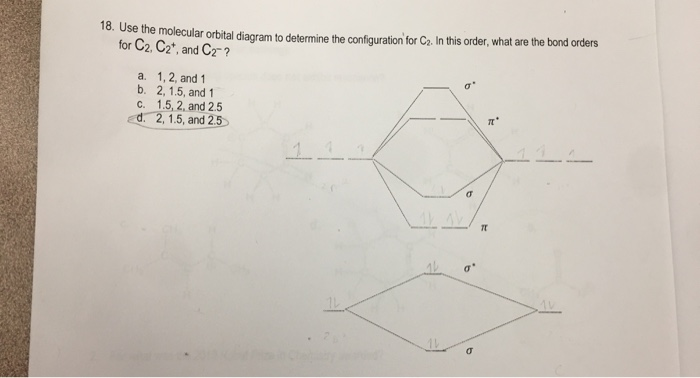
The molecular orbital diagram for #C_2# Chemistry Molecular Orbital Theory Molecular Orbital Theory. 1 Answer Stefan V. Dec 2, 2016 Here's what I got. Explanation: The problem provides you with the MO diagram for the #"C"_2# molecule, so all you really have to do here is add an electron to that diagram. You need to ...
Molecular Orbital Theory. The Valence Bond Theory fails to answer certain questions like why He 2 molecule does not exist and why O 2 is paramagnetic. Therefore in 1932 F. Hood and R.S. Mulliken came up with Molecular Orbital Theory to explain questions like the ones above.
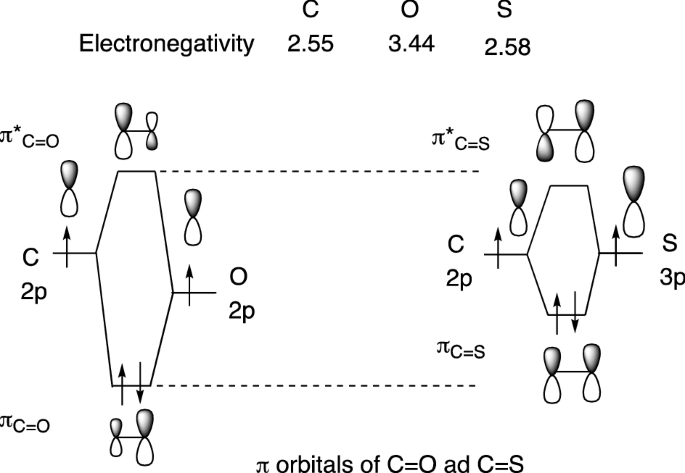
orbitals are used. So CS is like C≡O except you use 3s,3p orbitals for . the example thus ignores 1s on C and 1s,2s,2p on S Similar point, Cl uses 3s,3p and Br uses 4s,4p. Cl (right) more electronegative, so orbitals lower in energy than Br (left) orbitals are from interaction of sp hybrids, make linear C-C bonds, however the diagram
Energy level diagram for the molecular orbitals of OH ). H and O atom orbitals, which combine to form the molecular orbitals, are on the left and right side of the figure, respectively.
This section contains the course materials for Unit II, including lecture videos, readings, lecture notes, and practice problems.
atomic orbitals . C2v E C2 σv(xz) σv' (yz) A1 1 1 1 1 z x2, y2, z2 A2 1 1 -1 -1 Rz xy B1 1 -1 1 -1 x, R y xz B2 1 -1 -1 1 y, R x yz C3v E 2C 3 3σv A1 1 1 1 z x2 + y2, z2 A2 1 1 -1 Rz E 2 -1 0 (x,y), (R x, R y) (x 2-y2, xy)(xz, yz) •Symmetry elements possessed by the point group are in the top row •Left hand column gives a list ...
Answer (1 of 5): The total number of electron of CN- ion is ( 6+7+1) = 14 . According to molecular orbital theory, the electronic configuration of CN - ion is as follows, From the above electronic configuration , it has been found that , the number of bonding electron is 10 and the the number of...
January 28, 2017 - Molecular orbital : Stability of molecule is determined by bond order.Higher is the Bond order greater is the stability of molecule.
November 24, 2020 - IFigure \(\PageIndex{2}\): Molecular Orbital Energy-Level Diagram for \(\pi\) Bonding in Ethylene. As in the diatomic molecules discussed previously, the singly occupied 2pz orbitals in ethylene can overlap to form a bonding/antibonding pair of \(\pi\) molecular orbitals.
ORBITALS - are specific regions of space where electrons may exist - The SHAPE of an orbital is defined by the SUBSHELL it is in - The ENERGY of an orbital is defined by both the SHELL the orbital is in AND the kind of SUBSHELL it is in ARRANGEMENT OF SHELLS, SUBSHELLS, AND ORBITALS - Shells are numbered.
Let us know see how we can draw the Lewis Structure for CS2. 1. Carbon belongs to Group 4 of the periodic table. Therefore, the number of valence electrons in the Carbon atom =4. Sulfur (S) belonging to Group 6 has 6 valence electrons. CS2 has two S atoms, hence, the valence electrons in sulfur here are 6*2=12.
Carbon monosulfide. Formula: CS. Molecular weight: 44.076. IUPAC Standard InChI: InChI=1S/CS/c1-2. Copy Sheet of paper on top of another sheet. IUPAC Standard InChIKey: DXHPZXWIPWDXHJ-UHFFFAOYSA-N. Copy Sheet of paper on top of another sheet. CAS Registry Number: 2944-05-.
A partial Mo (molecular orbitals) diagram for CS is shown on the picture. a) Considering only electronegativity values, place the 3p atomic orbital for sulfur in the correct location. [Select] Answer Bank b ь b) In this diagram, which orbital is the LUMO? [Select] b) In this diagram, which orbital is the LUMO?
The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 8). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one ...
Steps to form OF2 Lewis Structure Diagram. Step 1: Find the Total number of Valence Electrons. The first and foremost step is to calculate the total number of valence electrons in an OF2 molecule. Oxygen belongs to group 16, the chalcogen family, and has a valency of 6. Fluorine belongs to the family of halogen in group 17 and has a valency of 7.
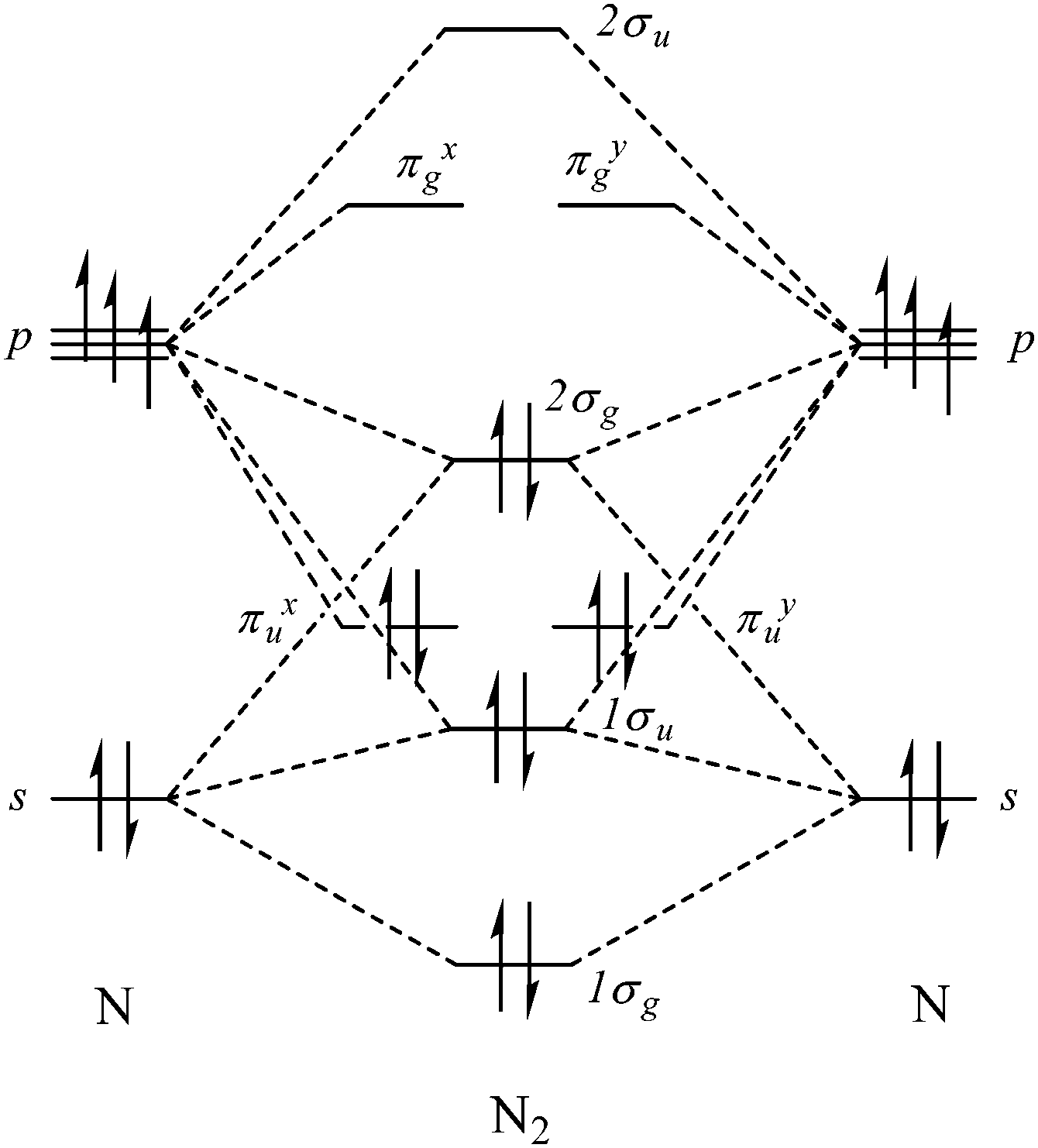
Mechanistic Aspects Of Dinitrogen Cleavage And Hydrogenation To Produce Ammonia In Catalysis And Organometallic Chemistry Relevance Of Metal Hydride Chemical Society Reviews Rsc Publishing Doi 10 1039 C3cs60206k
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that ...
Best Answer. This is the best answer based on feedback and ratings. 100% (34 ratings) Transcribed image text: Construct the molecular orbital diagram for N2 and then identify the bond order Bond order 0.5 O 1.5 O 2.5 2s 2s Click within the blue boxes to add electrons. Previous question Next question.
Solved Draw The Mo Diagram For Cs2 Showing All Of The Possible Orbitals For Each Atom Or Group Show Which Interactions Would Be Bonding Non Bond Course Hero
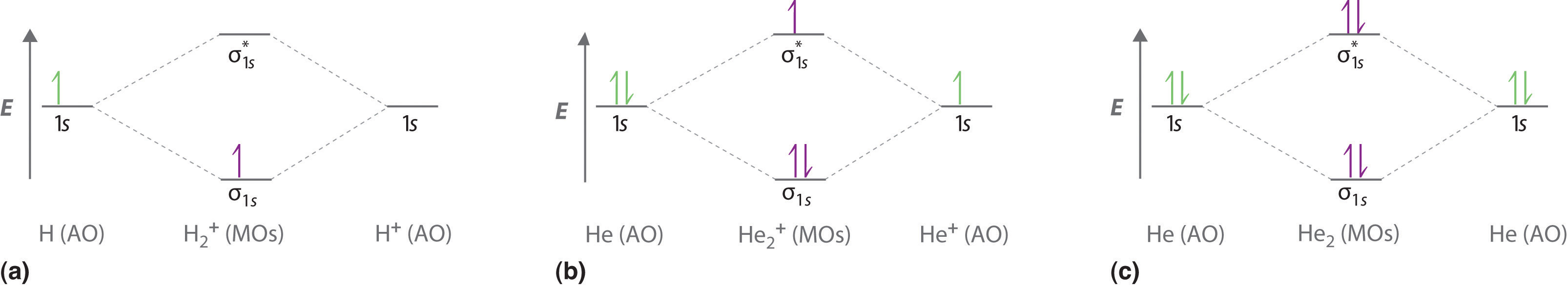
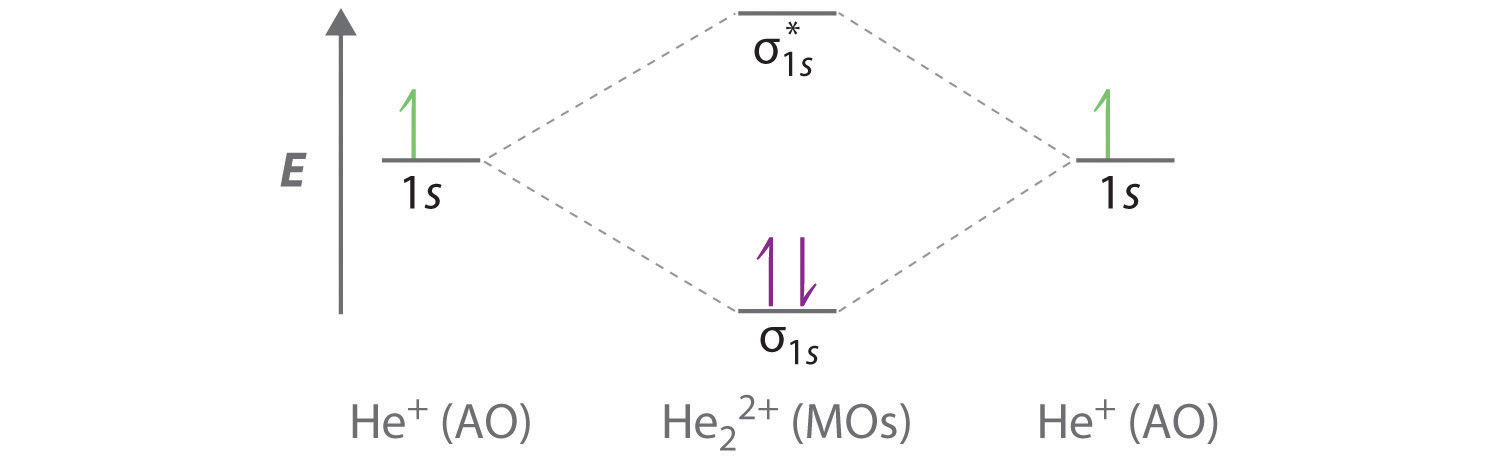
April 3, 2021 - This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H2+. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as ...
Download scientific diagram | Graphical representation of the LUMO orbitals for CO 2 , OCS and CS 2 calcu- from publication: Kinetics of the Reactions of Water, Hydroxide Ion and Sulfide Species ...
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we’ll see that symmetry will help us treat larger molecules in
Answer to Draw the MO diagram for CS2 . Showing the possible orbitals for each atom or group. Which would be bonding, non-bonding ...

Molecular Orbital Diagram White Png Download 745 572 Free Transparent Molecular Orbital Diagram Png Download Cleanpng Kisspng












0 Response to "36 cs molecular orbital diagram"
Post a Comment