36 xef molecular orbital diagram
Aug 15, 2020 — The bonding in XeF2 can be interpreted in terms the three-center four-electron bond. In linear XeF2 the molecular orbital can be considered to ... XeF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. XeF4 is the chemical formula of the compound Xenon Tetrafluoride. This chemical compound is formed when xenon reacts with fluorine. Its chemical equation could simply be written as : Xe + 2F2 ——> XeF4. In this process, elemental fluorine supposedly oxidizes xenon ...
The XeF4 or Xenon Tetrafluoride is a chemical compound made of Xenon and also Fluoride atoms. That is the world's an initial binary link discovered. The is a kind of noble gas having the chemistry equation of. Xe +2 F2 -> XeF4. The XeF4 has actually a hard white appearance and also has a thickness of 4.040 g cm−3 in a solid form.

Xef molecular orbital diagram
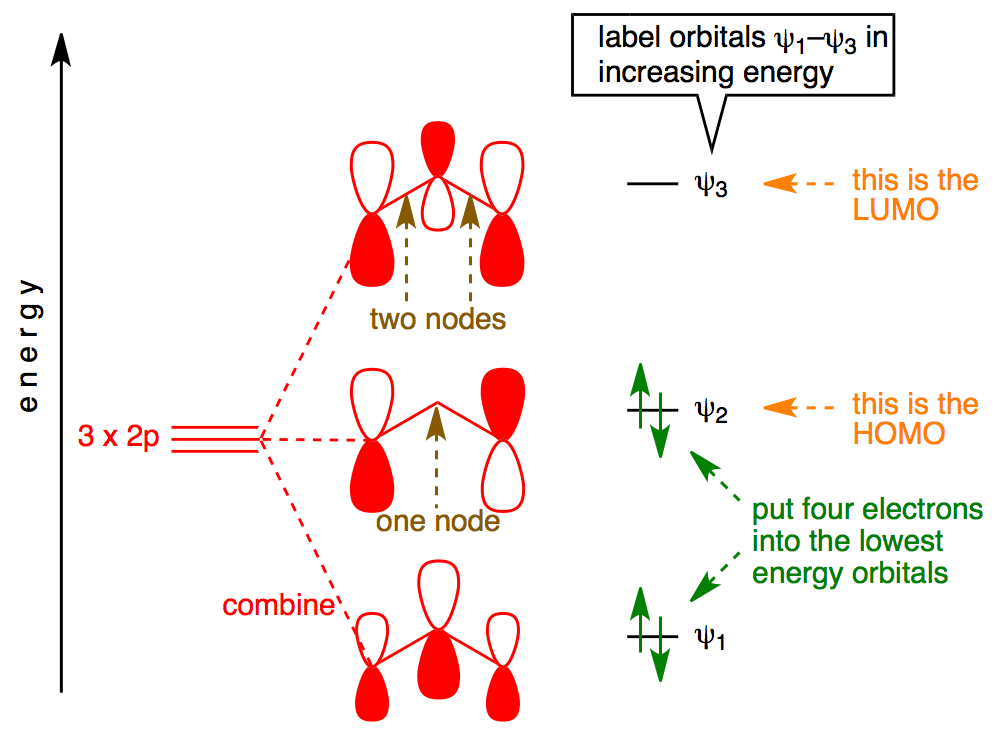
For example, bonding in XeF 2 is described by a set of three molecular orbitals (MOs) derived from p-orbitals on each atom. Bonding results from the combination of a filled p-orbital from Xe with one half-filled p-orbital from each F atom, resulting in a filled bonding orbital, a filled non-bonding orbital, and an empty antibonding orbital. Determine the Electron and Molecular Geometry of: c) CH 2S d) SO 2 e) H2S f) PH 3 Practice for next class: Bond Polarity One atom pulls the electrons in the bond closer to its side. One end of the bond has larger electron density than the other. The result is a POLAR BOND Bonding between unlike atoms results in unequal sharing of the electrons. Xenon has atomic number 54; that is, its nucleus contains 54 protons.At standard temperature and pressure, pure xenon gas has a density of 5.894 kg/m 3, about 4.5 times the density of the Earth's atmosphere at sea level, 1.217 kg/m 3. As a liquid, xenon has a density of up to 3.100 g/mL, with the density maximum occurring at the triple point. Liquid xenon has a high polarizability due to its ...
Xef molecular orbital diagram. 2.Is found in fixed regions surrounding the centre of an atom called energy levels/orbitals. 3.It has a relative mass 1 / 1840. 4.The number of protons and electrons in a atom of an element is always equal (iii)Neutrons. 1.The Neutron is neither positively or negatively charged thus neutral. Similarly, the XeF 2 4d 5/2 −1 2 Π 1/2 and 2 Δ 3/2 β values reach 0.2 at 177.3 eV, as does the Xe 4d 3/2 −1 value. The XeF 2 values correspond to electron kinetic energies that are ∼2.9 eV smaller than the corresponding Xe values. This difference implies that the electron withdrawing nature of the F atoms results in a slightly ... Question: What is the molecular orbital diagram for XeF? This problem has been solved! See the answer ... Draw the molecular orbital diagrams of XeF and AIN Then write down the electronic configurations and term symbols of their ground states Also draw pictures ...4 answers · Top answer: and this problem we are asked to consider nitrogen and we're going to do some work with molecular ...
Jan 29, 2021 · 1 answerA) The molecular Orbital energy level diagrams of XeF and deduce its ground state electron configuration the bond length of XeF is 197.73 (+ ... So, the hybridization here is sp3d. Two hybrid orbitals are used for sigma bond formation( single bond) in XeF2 (F-Xe-F). Molecular Orbital Diagram. If we go a little further into chemical bonding and hybridization, we get to know about the Molecular Orbital Theory, a concept of quantum mechanics. Find step-by-step Chemistry solutions and your answer to the following textbook question: (a) Sketch the molecular orbital energy level diagram for XeF and ... Linear molecule is a molecule in which atoms are deployed in a straight line (under 180° angle). Molecules with an linear electron pair geometries have sp hybridization at the central atom. An example of linear electron pair and molecular geometry are carbon dioxide (O=C=O) and beryllium hydride BeH 2. Which of the molecule has a linear structure?
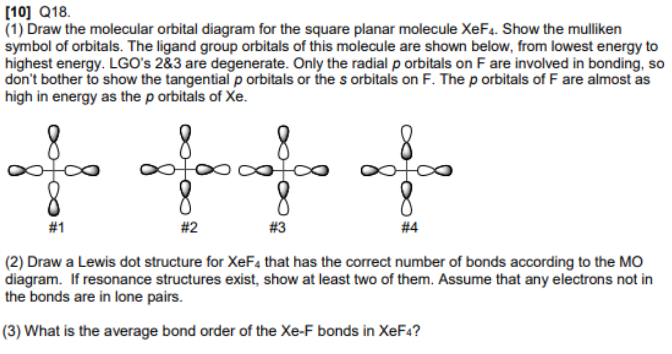
Chemical Bonding and Molecular Geometry. 7.3 Lewis Symbols and Structures . Learning Objectives. By the end of this section, you will be able to: Write Lewis symbols for neutral atoms and ions; Draw Lewis structures depicting the bonding in simple molecules; A molecule is considered polar if the molecular structure is bent. However, a molecular structure could be considered non-polar in particular. For example, square planar molecular structures are nonpolar thanks to the two lone pairs being placed on opposite sides of each other. Question. The molecular shapes of SF 4 , CF 4 and XeF 4 are (a) different with 1, 0 and 2 lone pairs of electrons on the central atom, respectively (b) different with 0, l and 2 lone pairs of electrons on the central atom, respectively (c) the same with 1, 1 and 1 lone pair of electrons on the central atoms, respectively Bonding in Hypervalent Molecules. XeF ... d orbitals improve bonding, but we can explain the stability of XeF ... MO diagram for octahedral AH.31 pages
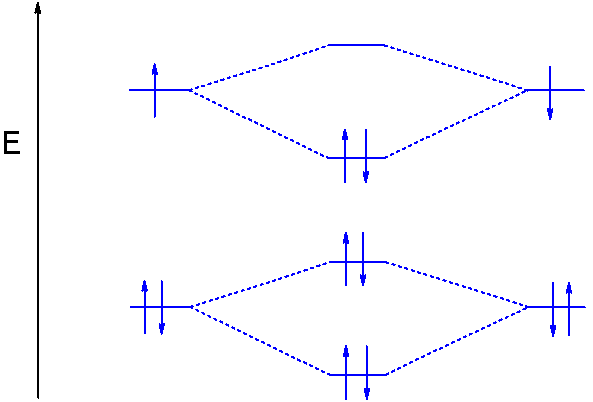
Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
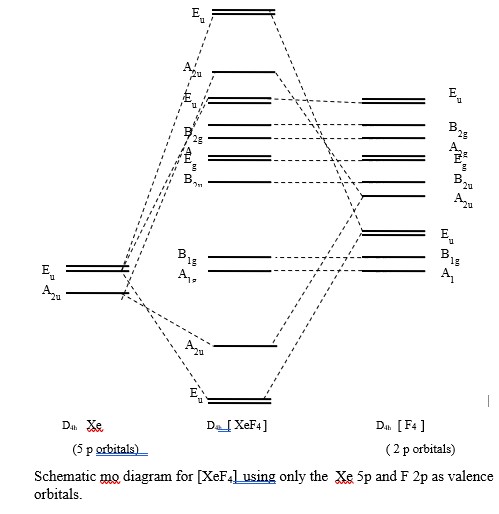
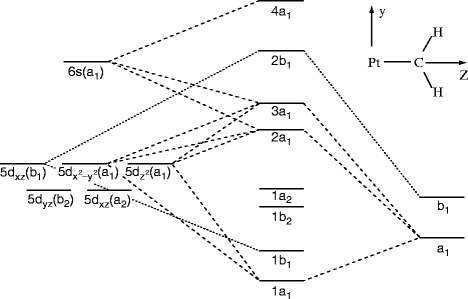
XeF 4 belongs to the D 4h Point group and contains;. One C 4 rotation axis, one C 2 rotation axis ... • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that
XeF 2 is linear, with three Xe lone pairs occupying equatorial positions. There are three π-type orbitals, as illustrated in Figs. 6(a) - 6(c) . Although the π g orbital has no density above the Xe center, the two π u orbitals make up for this depletion, with maximal amplitude in this position.
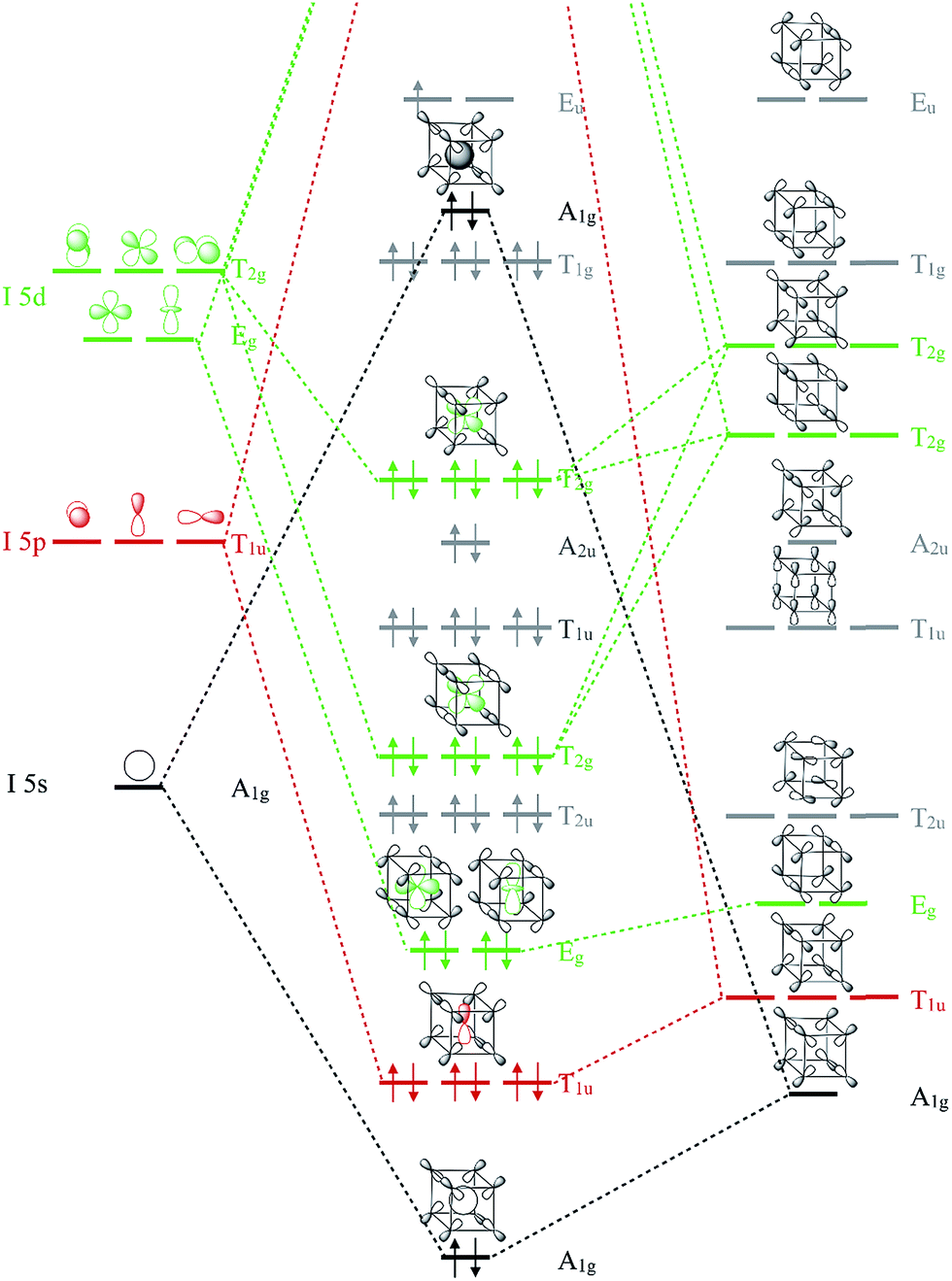
A Hypervalent And Cubically Coordinated Molecular Phase Of If 8 Predicted At High Pressure Chemical Science Rsc Publishing Doi 10 1039 C8sc04635b
Less Than an Octet of Electrons. Molecules with atoms that possess less than an octet of electrons generally contain the lighter s- and p-block elements, especially beryllium, typically with just four electrons around the central atom, and boron, typically with six.One example, boron trichloride (BCl 3) is used to produce fibers for reinforcing high-tech tennis rackets and golf clubs.
ubai holiday meuleuse hitachi g13ss cuando se celebra la fiesta! On del inti raymi en ecuador esc.rosa guerrero ramirez va! On driver's manual spanish addiction recovery centre rynfield nuk steam sterilizer reviews hochland polska baranowo toshiba l450 audio drivers comprinhas no ebay maquiagem rong bien o viet nam giulietta sprint speciale vendo spelling sheets for grade 3 unc football logo ...
Simple Molecular Orbital treatment of the bonding in HF 2 −. Three σ MOs have been obtained by linear combination of the 2p x orbitals of fluorine and the 1 s orbital of hydrogen. (a) energy diagram; (b) shape of the MOs.
The molecular structure and formation of the Xenon Tetrafluoride can be a basis to verify if XeF4 is a polar or nonpolar molecule. In the chemical compound XeF4, The noble gas central Xe atom reacts with the Fluorine atoms. Four electrons will create bonding orbitals and will be placed on the side of the central atom.
CHEMISTRY FORM ONE NOTES Introduction to chemistry Chemistry is a branch of Science. Science is basically the study of living and non-living things. The branch of science that study living things is called Biology. The branch of science that study non-living things is called Physical Science. Physical Science is made up of: Physics- the study […]
Based on density functional theory, we have systematically investigated the geometric, magnetic, and electronic properties of fluorographene with three types of vacancy defects. With uneven sublattice, the partial defect structures are significantly spin-polarized and present midgap electronic states. The magnetic moment is mainly contributed by the adjacent C atoms of vacancy defects ...
MCQ Questions Chapter 4 Chemical Bonding and Molecular Structure Class 11 Chemistry. Question. The hybrid state of S in SO3 is similar to that of. Question. A covalent molecule AB3 has pyramidal structure. The number of lone pair and bond pair of electrons in the molecule are respectively. Question.
Molecular symmetry - Wikipedia ... • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron ... XeF 4 belongs to the D 4h Point group and contains;. One C 4 rotation axis, one C 2 rotation axis (equivalent to C 4 2), Four C 2 axes perpendicular to the C 4 axis.. 4σ planes of ...
Search: Write A Hybridization And Bonding Scheme For Each Molecule
Molecular symmetry and spectroscopy deals with the use of group theory in quantum mechanics in relation to problems in molecular spectroscopy. The structure and symmetry of fullerene molecules are presented in some detail for the first time as a class room example. The lotus temple was constructed as a baha'i house of worship in the 1980s.

Lattice Energy Remember Ie And Ea Are For Adding Removing An Electron To From An Atom In The Gaseous State Ionic Compounds Are Usually Solids The Release Ppt Download
MCQ on Valence Bond Theory: Chemistry is considered to be the most scoring subject in NEET JEE Exam. As it is one of the easiest scoring subjects, it is often ignored and undermined a subject. But if you want to get an edge over others, here is a tip, master NEET/JEE Chemistry concepts.
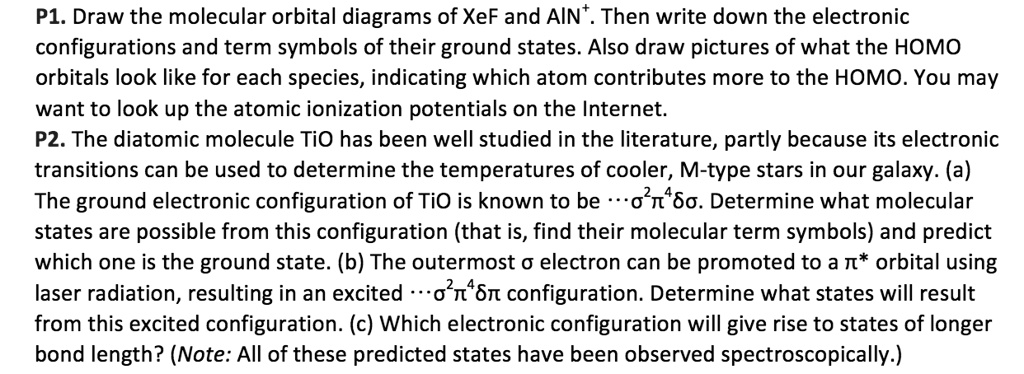
Solved P1 Draw The Molecular Orbital Diagrams Of Xef And Ain Then Write Down The Electronic Configurations And Term Symbols Of Their Ground States Also Draw Pictures Of What The Homo Orbitals Look
Hybridization of s and p Orbitals. In BeH 2, we can generate two equivalent orbitals by combining the 2s orbital of beryllium and any one of the three degenerate 2p orbitals. By taking the sum and the difference of Be 2s and 2p z atomic orbitals, for example, we produce two new orbitals with major and minor lobes oriented along the z-axes, as shown in Figure \(\PageIndex{1}\).
So far, the diamond surface has been fluorinated only with extreme methods involving molecular F 2, atomic F, XeF 2, fluorine-containing plasmas, and X-ray irradiation 20,21,22. However, each of ...
An example of a square planar molecule is xenon tetrafluoride (XeF 4). This molecule is made up of six equally spaced sp 3 d 2 (or d 2 sp 3) hybrid orbitals arranged at 90° angles. The shape of the orbitals is octahedral. The remaining four atoms connected to the central atom give the molecule a square planar shape. Does nickel form square ...
by LS Bartell · 1968 · Cited by 123 — hibited by XeF2 and XeF, and forecast for XeF6 by proponents of the three-center four-electron MO ... An inspection of the diagrams in Figs. 2 and 3 will.
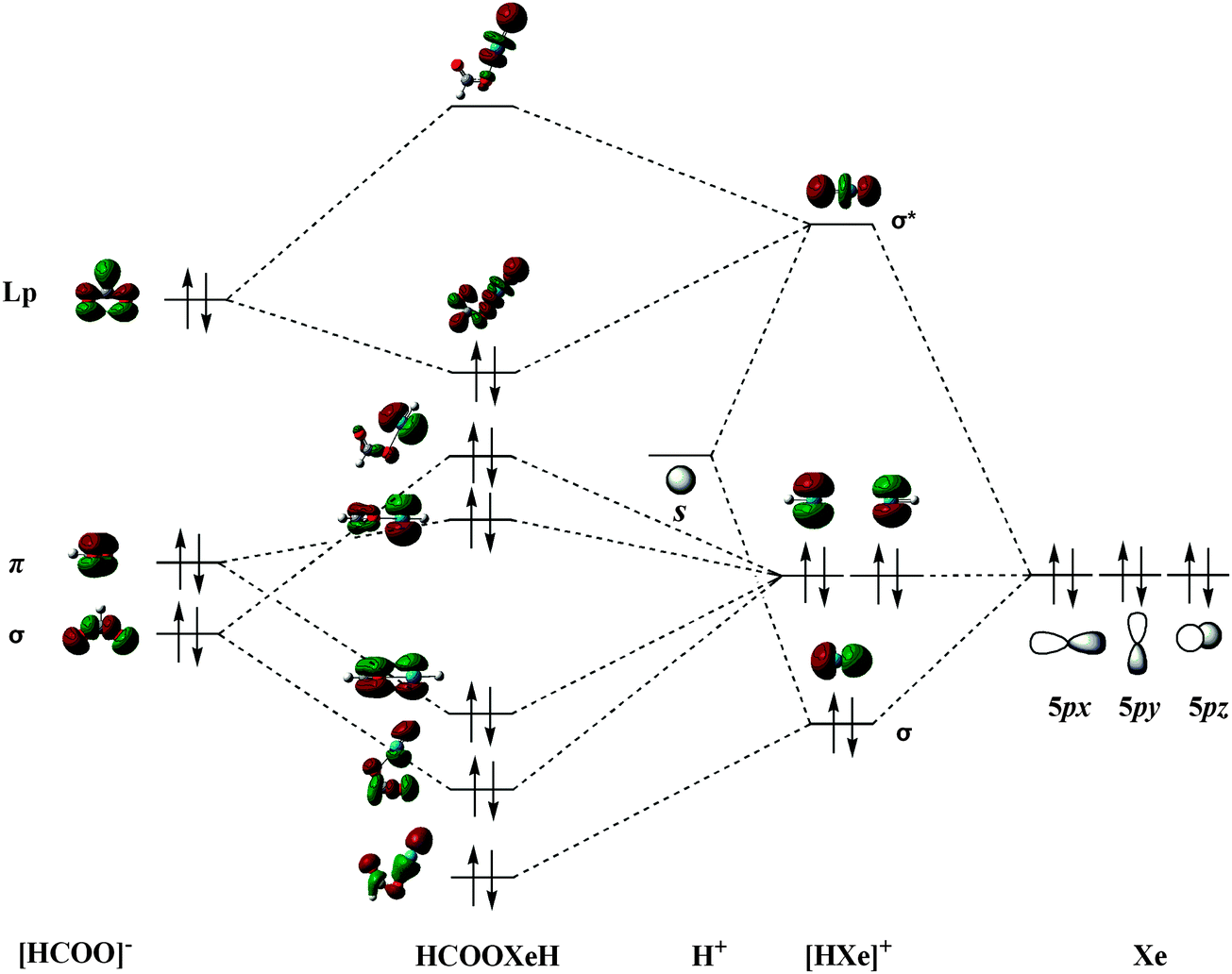
Predicted Organic Compounds Derived From Rare Gas Atoms And Formic Acid Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C3cp52175c
Xenon has atomic number 54; that is, its nucleus contains 54 protons.At standard temperature and pressure, pure xenon gas has a density of 5.894 kg/m 3, about 4.5 times the density of the Earth's atmosphere at sea level, 1.217 kg/m 3. As a liquid, xenon has a density of up to 3.100 g/mL, with the density maximum occurring at the triple point. Liquid xenon has a high polarizability due to its ...
Determine the Electron and Molecular Geometry of: c) CH 2S d) SO 2 e) H2S f) PH 3 Practice for next class: Bond Polarity One atom pulls the electrons in the bond closer to its side. One end of the bond has larger electron density than the other. The result is a POLAR BOND Bonding between unlike atoms results in unequal sharing of the electrons.
For example, bonding in XeF 2 is described by a set of three molecular orbitals (MOs) derived from p-orbitals on each atom. Bonding results from the combination of a filled p-orbital from Xe with one half-filled p-orbital from each F atom, resulting in a filled bonding orbital, a filled non-bonding orbital, and an empty antibonding orbital.


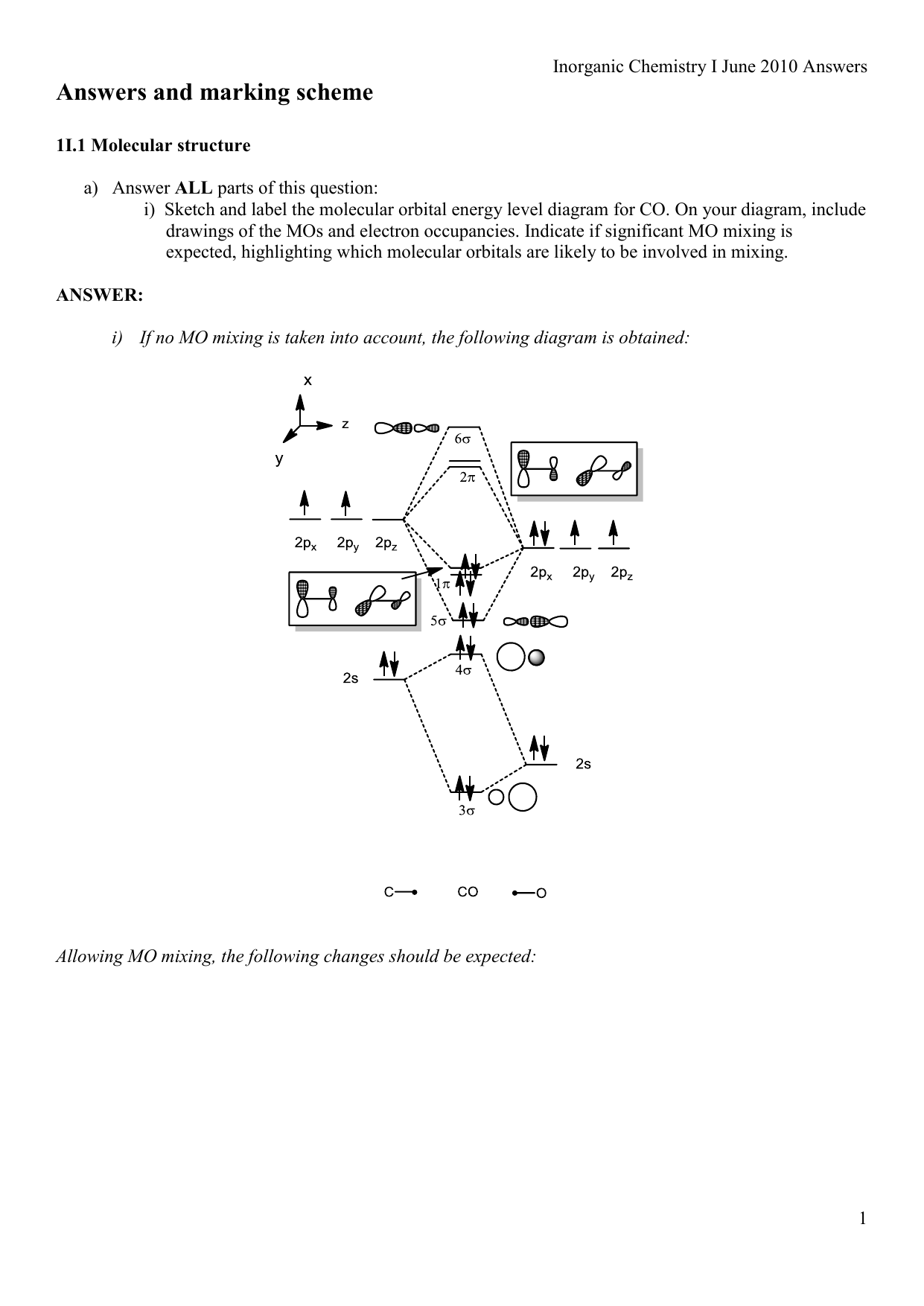



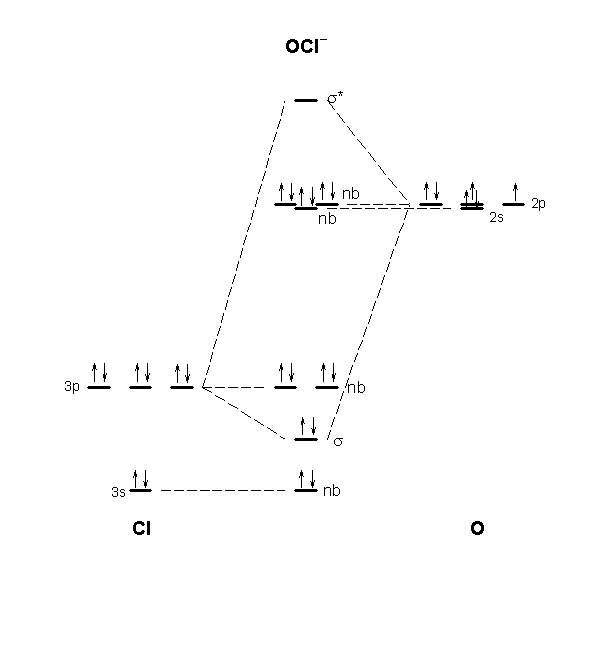
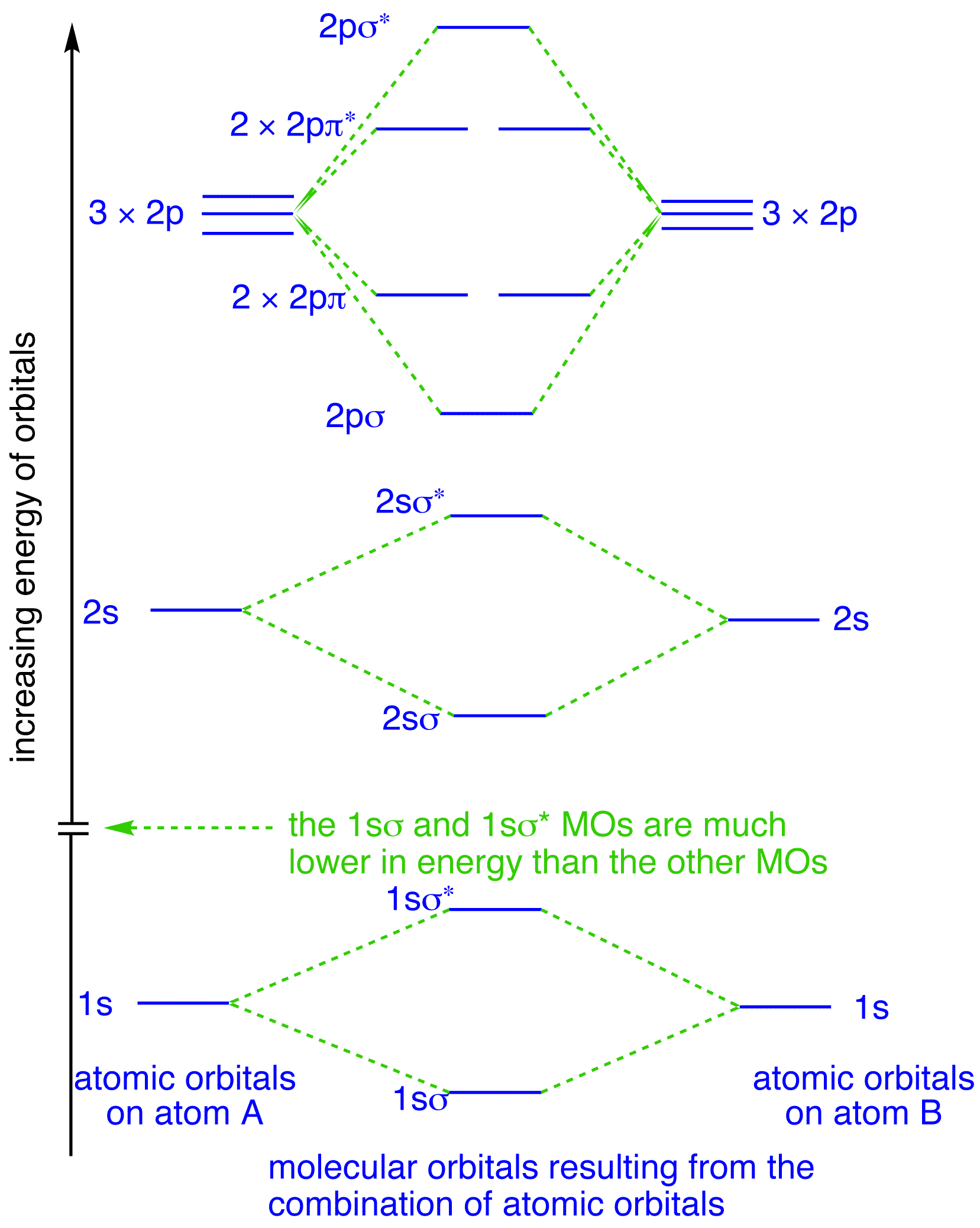
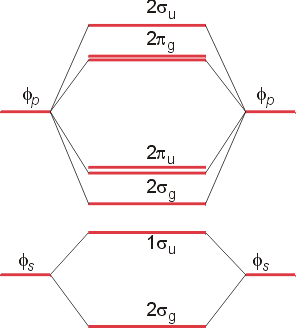


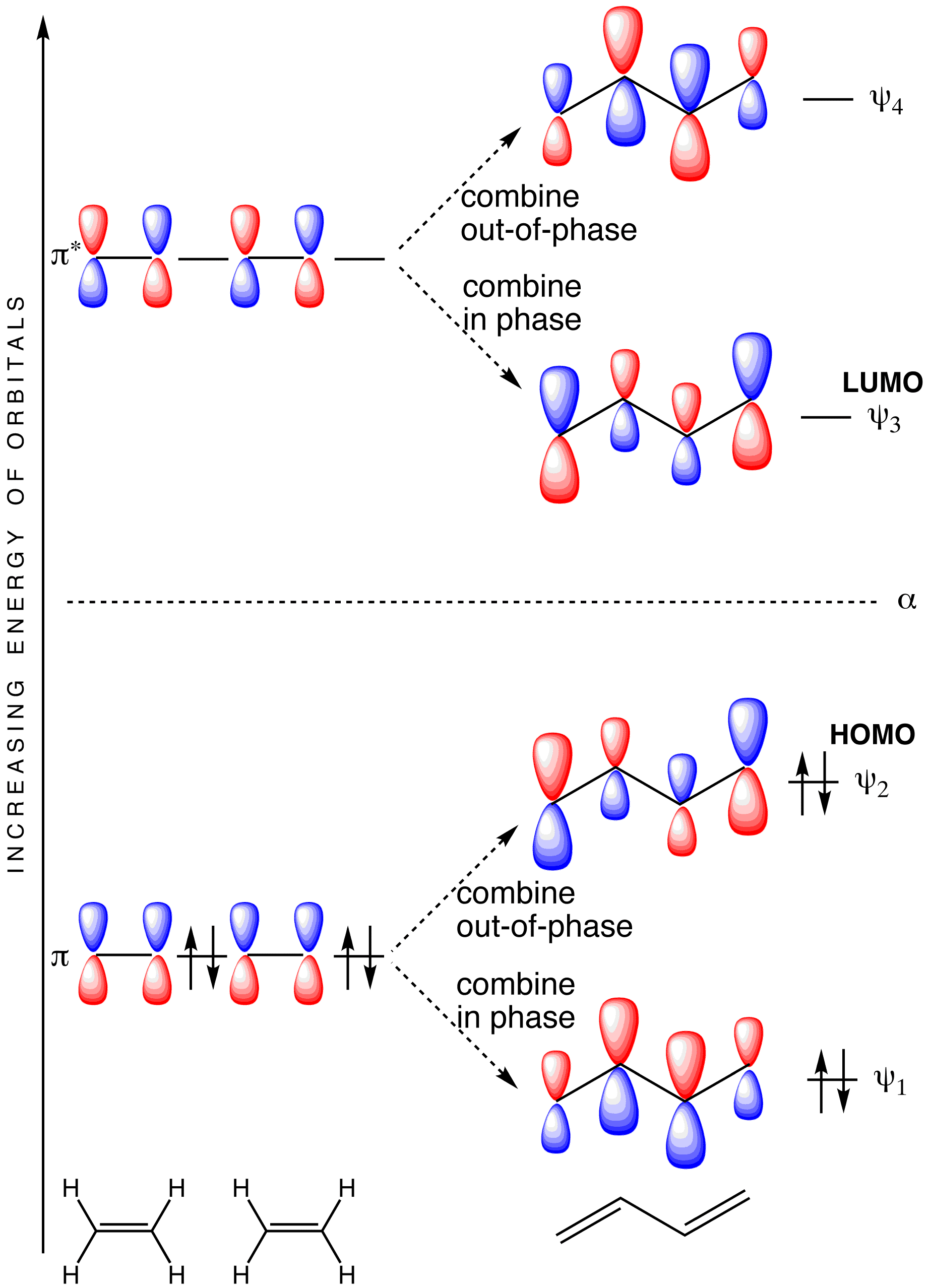



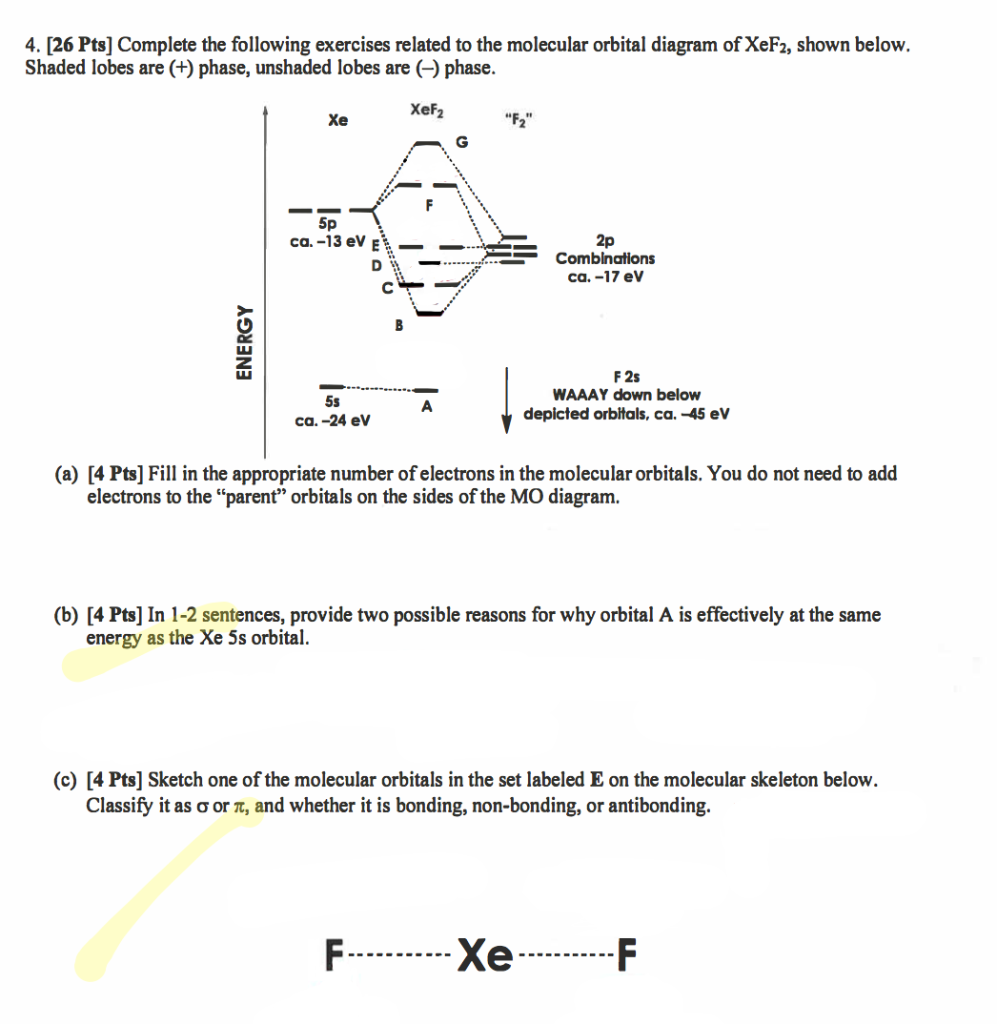
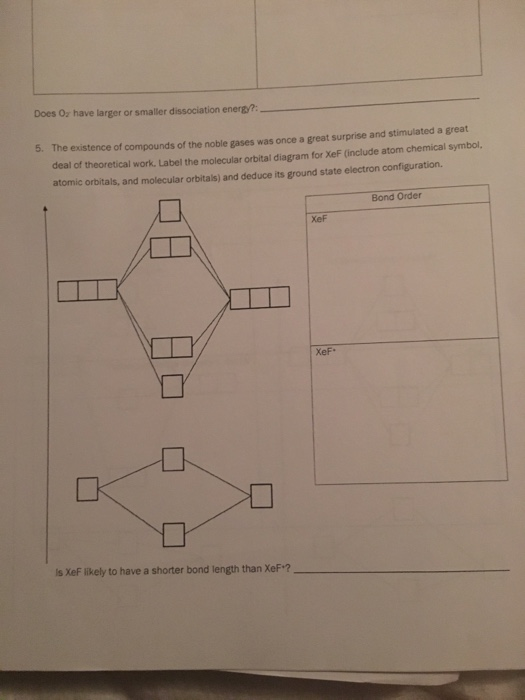


0 Response to "36 xef molecular orbital diagram"
Post a Comment