37 frost diagram organic chemistry
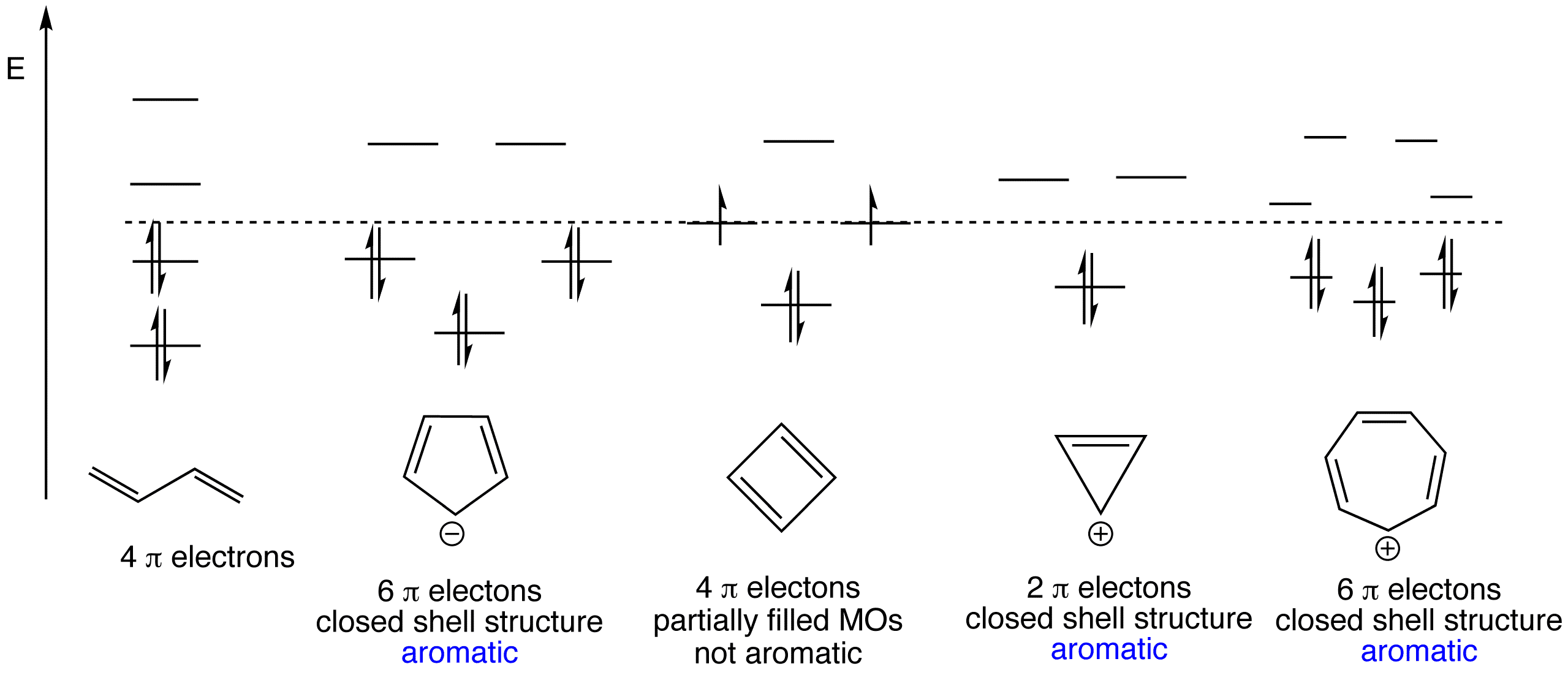
In organic chemistry, we will learn about the reactions chemists use to synthesize crazy carbon based structures, as well as the analytical methods to characterize them. We will also think about how those reactions are occurring on a molecular level with reaction mechanisms. Simply put, organic chemistry is like building with molecular Legos. Jan 29, 2019 · The Huckel aromaticity rules are: Molecule is cyclic. Have one pi orbital per atom of the ring. Planar, in an SP2 hybridized orbital, over every atom of the ring. Have a closed loop of 4n+2 pi-bond electrons, where n is equal to any integer (0,1,2,3,…) However, anti-aromatic compounds have an unusual INSTABILITY to them.
Laura D. Frost is an Associate Professor of Chemistry at Georgia Southern University, where she has taught chemistry to allied health students since 2000. She received her bachelor's degree in chemistry from Kutztown University and a Ph.D. in chemistry with a biophysical focus from the University of Pennsylvania.

Frost diagram organic chemistry
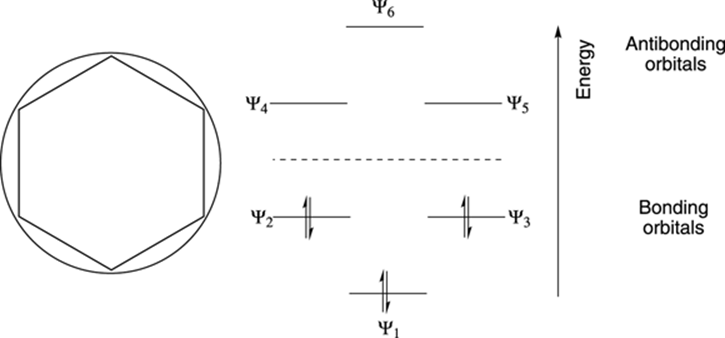
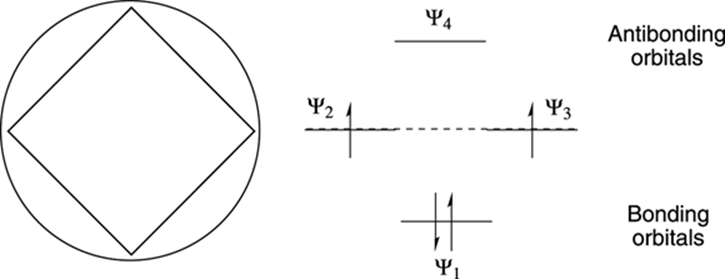
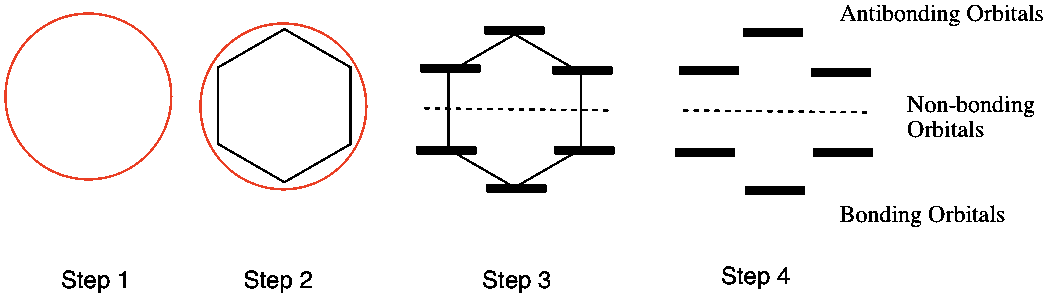
Frost Circles: relative energies of the molecular orbitals of cyclic, conjugated systems Inscribe the cyclic, conjugated molecule into a circle so that a vertex is at the bottom. The relative energies of the MO’s are where the ring atoms intersect the circle benzene: Benzene 6 !-electrons non-bonding level For aromatic compounds, such as benzene, Roger Frost's Organic Chemistry is fast, pin-sharp, full-screen animation showing addition, substitution, mechanisms, experiments, IR, NMR and more. It is made for teaching on a whiteboard and for students to revise with. It's a huge animation library, beautifully-organised by familiar headings in your exam. Frost Circles, and How To Use Them - Master Organic Chemistry. A Frost diagram or Frost-Ebsworth diagram is a type of graph used by inorganic chemists in electrochemistry to illustrate the relative stability of a number of different oxidation states of a particular substance. The graph illustrates the free energy vs oxidation state of a chemical species.
Frost diagram organic chemistry. Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a condenser. The vapours produced above the reaction continually undergo condensation, returning to the flask as a condensate. The reactants for reflux experiments can be solid and liquid, or ... Free download General, Organic and Biological Chemistry: An Integrated Approach (4th edition) written by Kenneth W. Raymond in pdf. This fourth edition of General, Organic, and Biological Chemistry: An Integrated Approach has, like the earlier editions, been written for students preparing for careers in health-related fields such as nursing, dental hygiene, nutrition, occupational therapy ... Video explaining Frost Circle for Organic Chemistry. This is one of many videos provided by Clutch Prep to prepare you to succeed in your college classes. Subjects . ... For the energy diagram shown below: 1) There are 3 antibonding orbitals and 2 bonding orbitals 2) There are 2 antibonding orbitals and 2 non-bonding orbitals and 1 bonding ... Download General, Organic, & Biological Chemistry Book For Free in PDF, EPUB. In order to read online General, Organic, & Biological Chemistry textbook, you need to create a FREE account. Read as many books as you like (Personal use) and Join Over 150.000 Happy Readers. We cannot guarantee that every book is in the library.
Juhee Kim, Seongwon Yoon, Kyu Min Sim, Dae Sung Chung, Rational design of a junction structure to realize an NIR-selective narrowband organic thin-film photodiode, Journal of Materials Chemistry C, 10.1039/C9TC00722A, (2019). Resonance Structure The Kekulé structure would have the single bonds of longer length than the double bonds, and thus an irregular hexagonal shape. But spectroscopy had shown that benzene had a planar ring, with all the carbon-carbon bond distances the same 1.397Å (C-C typically 1.48Å, C=C typically 1.34Å). Inorganic Chemistry (Atkins, Shriver).PDF. Luedu Jkdhask. Download Download PDF. Full PDF Package Download Full PDF Package. This Paper. A short summary of this paper. 34 Full PDFs related to this paper. Read Paper. Download Download PDF. Aromatic or not: The Frost Circle. According to Hückel's rule, an aromatic compound must have (4n + 2) π electrons that form an uninterrupted π electron cloud. In addition, it must be planar and cyclic. If the compound is not planar and cyclic then it is also not aromatic. The bonding π MO (molecular orbitals) of an aromatic compound are ...
Levels of Protein Structure. The structure of proteins is generally described as having four organizational levels. The first of these is the primary structure, which is the number and sequence of amino acids in a protein's polypeptide chain or chains, beginning with the free amino group and maintained by the peptide bonds connecting each amino acid to the next. [DIAGRAM] 2000 Nissan Frontier Manual Transmission Diagram pdf Download. November 13, 2021 Author: XAGC. [DIAGRAM] 2000 Nissan Frontier Manual Transmission Diagram Epub Download. Save Image. 32010 4S510 Genuine Nissan #320104S510 TRANSMISSION ASSY. How to use Frost Diagrams to predict the relative Molecular Orbital energies of aromatic and antiaromatic compounds. Also at the end looks at how to use sy... Graphics source: Wade, Jr., L.G. Organic Chemistry, 6th ed. Pearson Prentice Hall Inc., 2006 . 14 IR SPECTRUM OF AN ALCOHOL The most prominent band in alcohols is due to the O-H bond, and it appears as a strong, broad band covering the range of about 3000 - 3700 cm-1. The sheer size
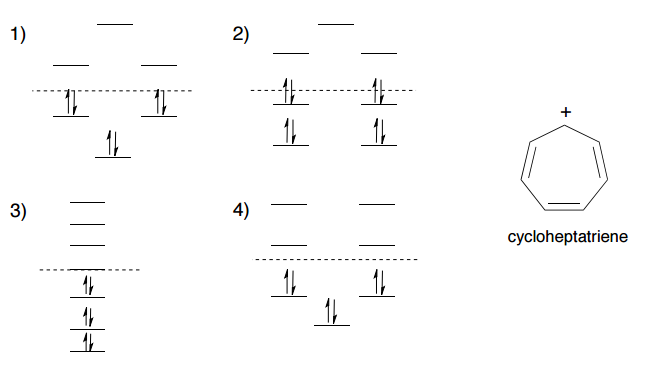
Organic Chemistry I Lecture 25 Organic Chemistry 1 Professor Duncan Wardrop April 6, 2010 1. ... Lecture 25: April 12 A. 8,3 B. 16, 10 C. 10, 3 D. 8,8 E. 8, 6 Below is the molecular orbital diagram for cyclooctatetraene; only the energy levels for each MO are indicated, not the MOs themselves. List the number ... Frost Circle: a mnemonic for ...
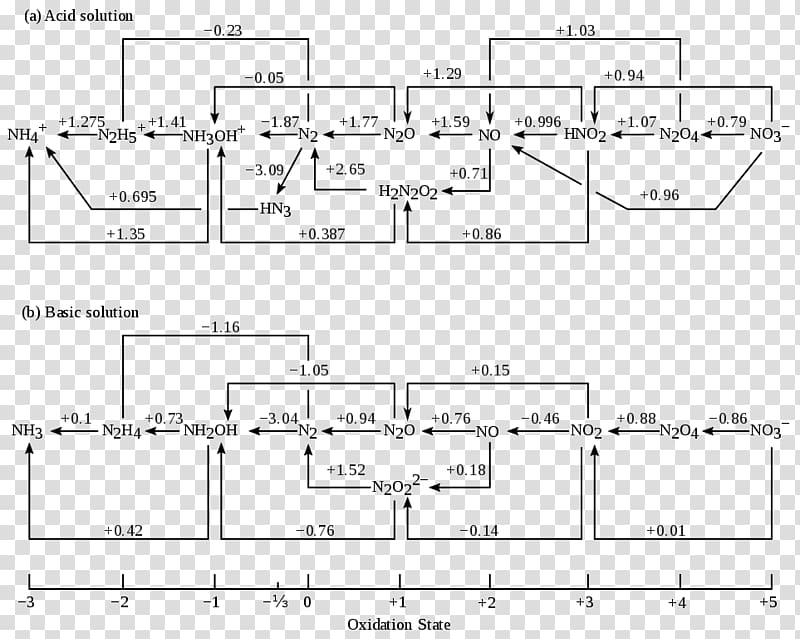
Frost diagrams are most useful as a qualitative description of the processes occurring, and for a quantitative description Latimer diagrams often prove more useful.The appearance of the Frost diagram leads to some useful features. The steeper the line joining two points in a Frost diagram, the higher the value of the reduction potential for the corresponding couple.
A Frost diagram or Frost–Ebsworth diagram is a type of graph used by inorganic chemists in electrochemistry to illustrate the relative stability of a number of different oxidation states of a particular substance. The graph illustrates the free energy vs oxidation state of a chemical species. This effect is dependent on pH, so this parameter also must be included. The free energy is determined by the oxidation–reduction half-reactions. The Frost diagram allows easier comprehension of ...
University Chemistry Textbooks (PDF Free Download) Here on college learner, you will have unlimited access to popular university chemistry textbooks from some of the best authors. You will also discover scholarly university chemistry textbooks. So, if you have been searching for answers on where to get: { college chemistry textbook pdf ...
Science · Organic chemistry ... So if I look at the dot structure, I can see that benzene has 2 pi electrons there, two here, and two more here, for a total of six pi electrons. If I look at the carbons of benzene, I can see that each carbon has a double bond to it. ... And the simplest way to do that is to draw a frost circle. And so here I ...
Frost Diagram Aromaticity Pdf. Written By JupiterZ August 29, 2017 Add Comment Edit. Diagramme De Phase H2o. Written By JupiterZ August 29, ... Line Diagram Organic Chemistry. Written By JupiterZ August 13, 2017 Add Comment Edit. Liquid Nitrogen Tank Diagram. Written By JupiterZ August 13, ...
INORGANIC CHEMISTRY-II BSCCH-201 UTTARAKHAND OPEN UNIVERSITY Page 2 (electropositive) and p-block (electronegative) elements, i.e. their properties have been found to be intermediate between those of the s-block and p-block elements. Thus these elements are located in the middle of the periodic table and are the
Sep 26, 2014 · Frost developed this mnemonic patterning as an extension of the Hückel ($4n+2$) rule. A Frost diagram is usually applied to all-carbon, monocyclic, π systems. It allows one to find the number of molecular orbitals in the molecule's π system and their energetic positions. To construct a Frost diagram, proceed as follows:
Visit Chapter-wise Courses for Preparation: https://vdnt.in/3fLy7PDF of Metallurgy Lecture 5 - https://drive.google.com/file/d/1Qoevh7F1zUxEZCbAOun1LeBKQnduR...
Aug 27, 2014 · Frost Diagrams are used to help draw the Molecular Orbitals for Valence Bond Structures. For example, say I have Benzene, and I want to know how many bonding electrons vs. non-bonding electrons it has. Our Valence Bond Structure looks like this: The first thing we do is draw the shape, with the point (any point) facing down.
5.61 Physical Chemistry Lecture #31 2 and πz orbitals that are odd with respect to reflection about z. These πz orbitals will be linear combinations of the pz orbitals on each carbon atom: z In trying to understand the chemistry of these compounds, it makes sense to focus our attention on these πz orbitals and ignore the σ orbitals. The πz
Frost circles are a useful trick for sketching out the pi molecular orbitals of cyclic pi systems. In this post we give many examples of how to make them. ... Your diagram of the cyclooctatetraene dianion shows the added electrons in a non-bonding orbital. With 10 electrons it would obey the numerology of Huckel’s rule but the diagram argues ...
By the way there's no real "reason" why this method works. It's just a coincidence (like how Bode's law describes the placement of planets in the solar system) that it describes this phenomenon so well. Tomorrow: we come back to carbocation stability.
2021-11-13. Create. 2005-08-08. Finely powdered native hydrous magnesium silicate. It is used as a dusting powder, either alone or with starch or boric acid, for medicinal and toilet preparations. It is also an excipient and filler for pills, tablets, and for dusting tablet molds. (From Merck Index, 11th ed)
Frost Circles, and How To Use Them - Master Organic Chemistry. A Frost diagram or Frost-Ebsworth diagram is a type of graph used by inorganic chemists in electrochemistry to illustrate the relative stability of a number of different oxidation states of a particular substance. The graph illustrates the free energy vs oxidation state of a chemical species.
Roger Frost's Organic Chemistry is fast, pin-sharp, full-screen animation showing addition, substitution, mechanisms, experiments, IR, NMR and more. It is made for teaching on a whiteboard and for students to revise with. It's a huge animation library, beautifully-organised by familiar headings in your exam.
Frost Circles: relative energies of the molecular orbitals of cyclic, conjugated systems Inscribe the cyclic, conjugated molecule into a circle so that a vertex is at the bottom. The relative energies of the MO’s are where the ring atoms intersect the circle benzene: Benzene 6 !-electrons non-bonding level For aromatic compounds, such as benzene,








![[Solved] Use a Frost circle to determine the Ï-electron ...](https://s3.amazonaws.com/si.question.images/image/images11/902-C-O-O-S(487).png)
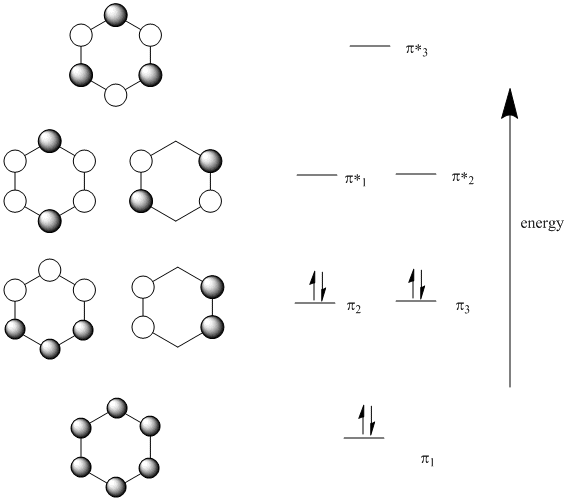



%2BL.%2BG.jpg)







0 Response to "37 frost diagram organic chemistry"
Post a Comment