37 in an electron dot diagram of ethylene how many double bonds are present
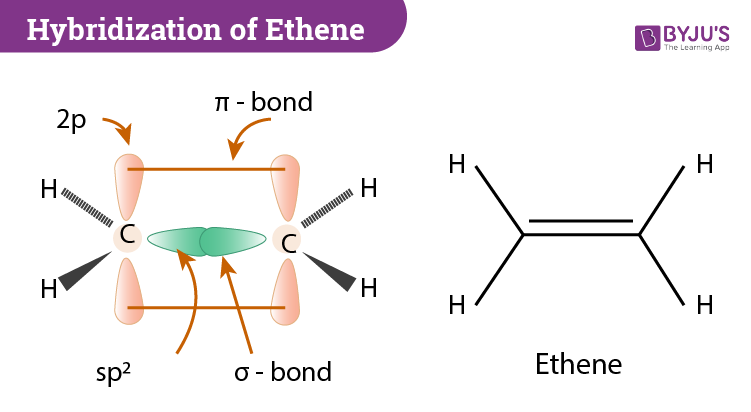
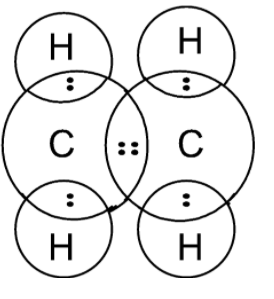
The single electrons match up to make pairs (Fig. 2.29 B). The oxygen atom forms two bonds, one with each of two hydrogen atoms; therefore, the formula for water is H 2 O. When an electron, or dot, from one element is paired with an electron, or dot, from another element, this makes a bond, which is represented by a line (Fig. 2.29 C). The three bonding regions form a trigonal planar electron-pair geometry. Thus we expect the σ bonds from each carbon atom are formed using a set of sp 2 hybrid orbitals that result from hybridization of two of the 2p orbitals and the 2s orbital . These orbitals form the C-H single bonds and the σ bond in the C=C double bond .
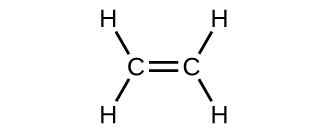
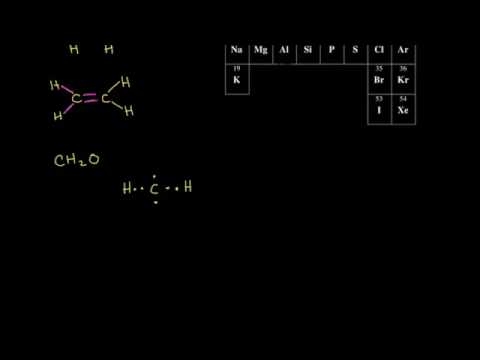
Structure and Bonding in Ethene: The. π. Bond. Ethene is the formal IUPAC name for H 2 C=CH 2, but it also goes by a common name: Ethylene. The name Ethylene is used because it is like an ethyl group ( C H 2 C H 3) but there is a double bond between the two carbon atoms in it. Ethene has the formula C 2 H 4 and is the simplest alkene because ...
In an electron dot diagram of ethylene how many double bonds are present
In an electron dot diagram of propane (C 3 H 8), how many double bonds are present? answer choices . three. two. one. none. Tags: Question 9 . SURVEY . 120 seconds . Q. Which of the following intermolecular forces plays a pivotal role in biological molecules such as proteins and DNA? 6 CHEM 1411. Chapter 7. Chemical Bonding I (homework) W a. I and II b. II, III, and IV c. III and IV d. II and III e. I, II, and III ____ 29. How many lone pairs of electrons are there on the S atom in the SCl 4 Hydrogen bonds can be found between molecules of which substance? NH3. Which compound contains a triple bond? acetylene (C2H2) In an electron dot diagram of ethylene (C2H4), how many double bonds are present? one. What is the molecular shape of silicon tetrabromide? tetrahedron.
In an electron dot diagram of ethylene how many double bonds are present. Since there are two bonds forming here, we will have a double bond structure. Hence, C2H4 is an alkene. Here, we have got the most suitable and appropriate Lewis Structure Sketch of ethylene. Molecular Geometry. When we draw the Lewis Structure of C2H4, we find a linear 2-D representation. In reality, the molecular shape of ethene is not linear. **The bonding π orbital is the lower energy orbital and contains both p electrons (with opposite spins) in the ground state of the molecule. The region of greatest probability of finding the electrons in the bonding π orbital is a region generally situated above and below the plane of the σ-bond framework between the two carbon atoms. In an electron dot diagram of propane (C3H8), how many double bonds are present?-three-none-one-two. none. What is the molecular shape of silicon tetrabromide?-tetrahedron-bent triatomic-pyramidal-linear. tetrahedron. Which intermolecular force plays a pivotal role in the unique properties of water? The formula is H 2 O so the dot and cross diagram includes two H atoms and one O atom. H has 1 outer electron. O has 6 outer electrons. The H circles must each overlap the O circle. Question Draw ...
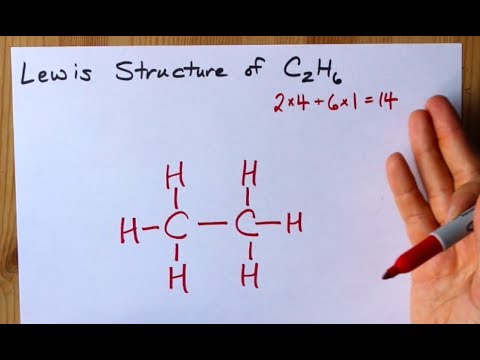
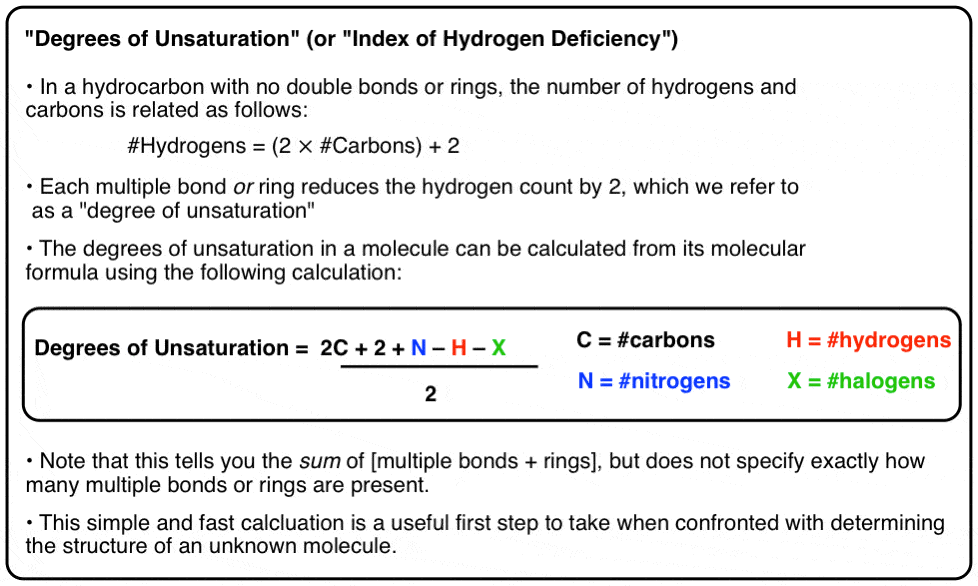
A simple view of double covalent bonds. A double covalent bond is where two pairs of electrons are shared between the atoms rather than just one pair. Some simple molecules containing double bonds. Oxygen, O 2. Two oxygen atoms can both achieve stable structures by sharing two pairs of electrons as in the diagram. C2H4 Lewis structure contains four C-H bonds and one double bond in between two carbon atoms. No lone pair is present on the central or outer atom in the lewis structure of C2H4. The lewis dot structure of C2H4 is very easy to draw-Some steps need to follow for drawing the C2H4 Lewis dot structure. 1. Count total valence electron in C2H4 In an electron dot diagram of ethylene (C2H4) how many double bonds are present?... What is the molecular shape of silicon tetrabromide? tetrahedron. Which of the following intermolecular forces plays a pivotal role in the unique properties of water? hydrogen-bonding. STEP 1: When it comes to finding the electron dot diagram of an ion you should first find it for its neutral form. Since the calcium atom is in Group 2A it will have 2 valence electrons. Lewis Dot Diagram (Calcium) STEP 2: Find the electron dot diagram of the cation. The 2+ charge means calcium has lost 2 valence electrons.
Ethylene, also known as ethene, has two carbon atoms and four hydrogen atoms. The carbon atoms in ethene exhibit a valency of 4; therefore, in order to complete their outermost shell, the carbon atoms form a double bond with one another. Thus, the number of double bonds present in a molecule of ethylene is 1. To count sigma and , first you need to know how many single, double and triple bonds are present in the molecule. A single bond contains one sigma bond (direct head-on overlap of orbitals). For example, the ##"C-C"## sigma bond in ethane is formed by the head-on overlap of two ##sp^3## orbitals. The C-H sigma […] Formula Lewis Electron-Dot Diagram : Ethanethiol CH 3 CH 2 SH : Ethane : CH 3 CH 3: Ethanol CH 3 CH 2 OH : Ethyne : C 2 H 2 (a) Draw the complete Lewis electron-dot diagram for ethyne in the appropriate cell in the table above. See the lower right cell in the table above. One point is earned for the correct Lewis structure. In an electron dot diagram of ethylene (C2H4) , how many double bonds are present? one. What is the molecular shape of silicon tetrabromide? tetrahedron. Which intermolecular force plays a pivotal role in the unique properties of water? hydrogen bonding. Which element does not form a monatomic ion?

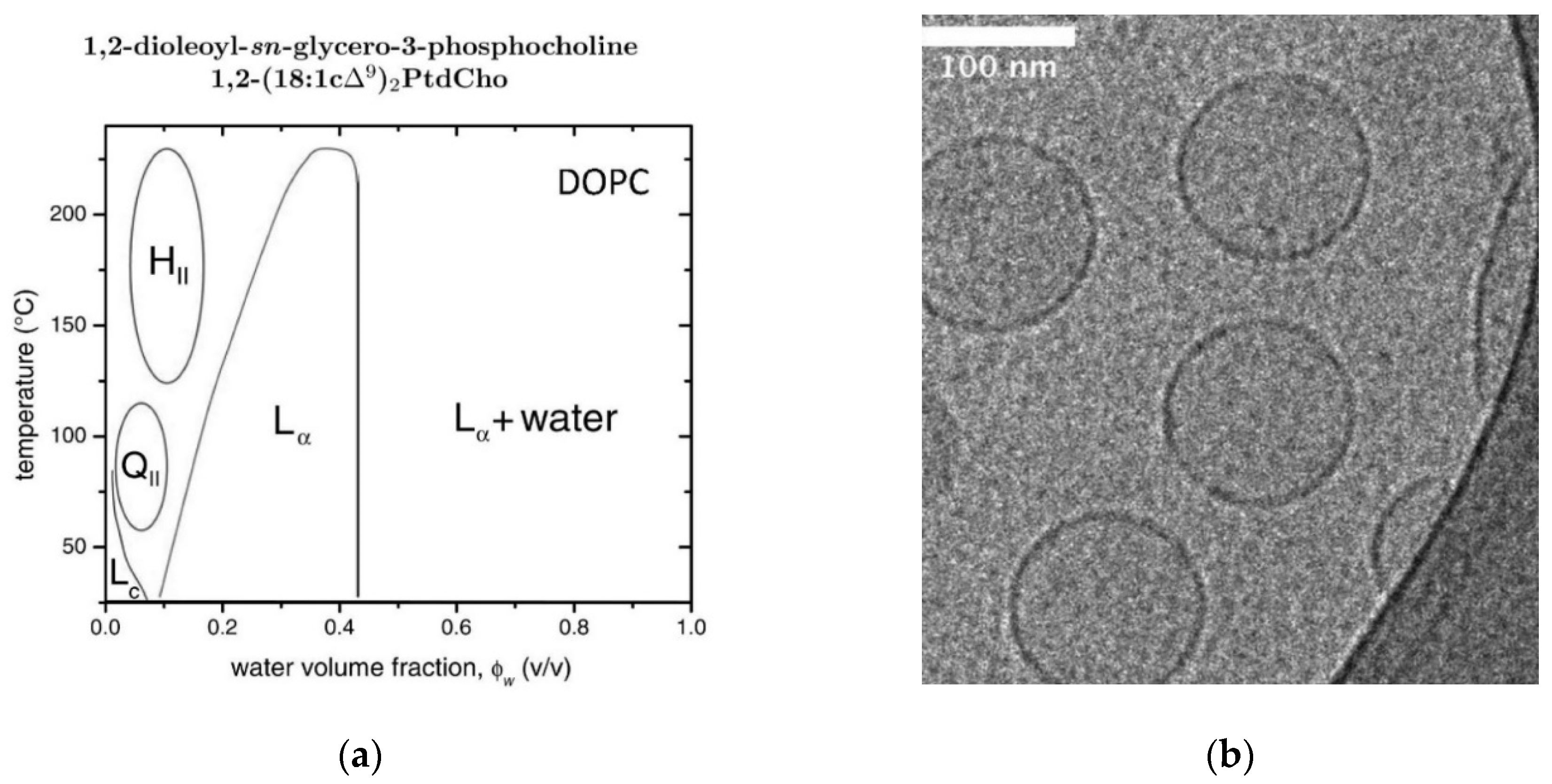
Applied Sciences Free Full Text Lipid Vesicles And Other Polymolecular Aggregates From Basic Studies Of Polar Lipids To Innovative Applications Html
In ethylene, for example, each carbon contributes two electrons to the double bond, giving each carbon an octet (two electrons/bond × four bonds = eight electrons). Neutral structures with fewer or more bonds exist, but they are unusual and violate the octet rule.
The elements that form strong double or triple bonds are C, N, O, P, and S. Because neither boron nor fluorine falls in this category, we have to stop with what appears to be an unsatisfactory Lewis structure. Too Many Electrons . It is also possible to encounter a molecule that seems to have too many valence electrons.
In an electron dot diagram of propane (c3h8), how many double bonds are present? onetwothreenone
Answer (1 of 6): There are 5 sigma bonds (strong) and 1 pi bond (weak) in ethene. There are 4 (C-H) bonds (sigma) and 1 (C-C) bond (sigma).The valency of carbon is 4. Each carbon atom is bonded to 2 hydrogen atoms and there is a sigma bond between the two carbon atoms. This accounts for the shar...
Draw the Lewis dot structure for CO. The number of valence electrons is 4 + 6 = 10 electrons or 5 pairs. Since both C and O allow multiple bonds we can still follow the octet and write: If there is not enough electrons to follow the octet rule, then the least electronegative atom is left short of electrons. Draw the Lewis dot structure for BeF2.
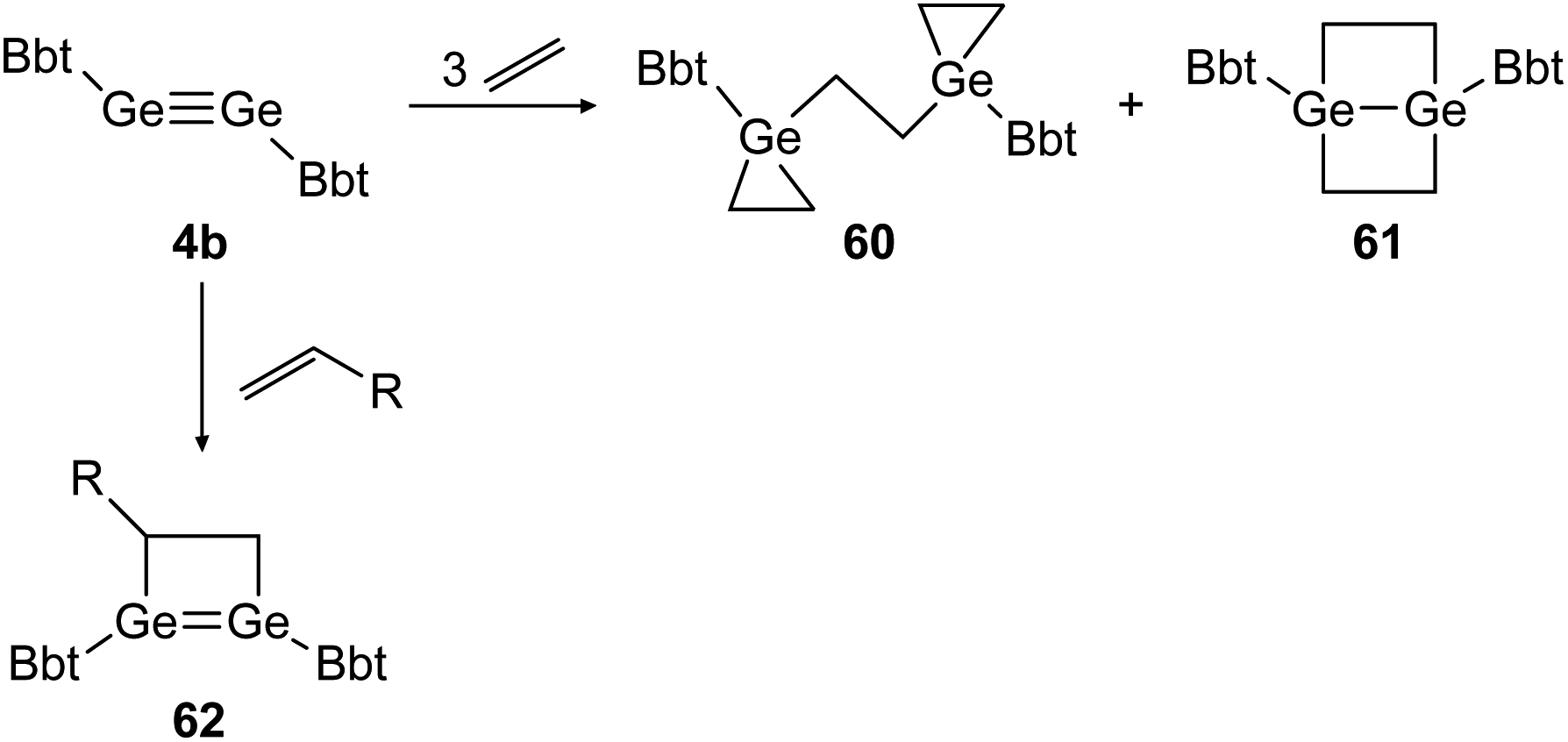
Recent Advances Of Group 14 Dimetallenes And Dimetallynes In Bond Activation And Catalysis Chemical Science Rsc Publishing Doi 10 1039 D0sc03192e
When double and triple bonds are present between two atoms, there is additional bonding holding the atoms together. While a sigma bond is always the first bond between two atoms, a pi bond is always the second bond between two atoms (…and third bond, if present). Pi bonds use 2p orbitals to overlap in
The bond consists of two electron clouds which lie above and below the plane of carbon and hydrogen atoms. (a) Formation of ethylene (b) Molecular orbital structure molecule of ethylene Thus, ethylene molecule consists of four sigma C - H bonds, one sigma C - C bond and one bond between carbon-carbon atom. The bond length of carbon-carbon ...
Answer : The number of double bonds present in propane is, Zero. Explanation : Lewis-dot structure : Lewis-dot structure shows the bonding between the atoms of a molecule and also shows the unpaired electrons present in the molecule. Carbon has '4' valence electrons and hydrogen has '1' valence electrons.
In this case, a single bond is formed between hydrogen and chlorine by sharing one electron. Double Bonds. A double bond is formed when two pairs of electrons are shared between the two participating atoms. It is represented by two dashes (=). Double covalent bonds are much stronger than a single bond, but they are less stable.

In Situ Drifts Investigation Of Ethylene Oxidation On Ag And Ag Cu On Reduced Graphene Oxide Springerlink
In an electron dot diagram of ethylene (C2H4), how many double bonds are present? One. What is the molecular shape of silicon tetrabromide? Tetrahedron. Which of the following intermolecular forces plays a pivotal role in biological molecules such as proteins and DNA? Hydrogen bonding.
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.

Molecules Free Full Text Insight Into Biomass Upgrade A Review On Hydrogenation Of 5 Hydroxymethylfurfural Hmf To 2 5 Dimethylfuran Dmf Html
Hydrogen bonds can be found between molecules of which substance? NH3. Which compound contains a triple bond? acetylene (C2H2) In an electron dot diagram of ethylene (C2H4), how many double bonds are present? one. What is the molecular shape of silicon tetrabromide? tetrahedron.
Review On Sensitive And Selective Ethylene Detection Methods For Fruit Ripening Application Emerald Insight
6 CHEM 1411. Chapter 7. Chemical Bonding I (homework) W a. I and II b. II, III, and IV c. III and IV d. II and III e. I, II, and III ____ 29. How many lone pairs of electrons are there on the S atom in the SCl 4
In an electron dot diagram of propane (C 3 H 8), how many double bonds are present? answer choices . three. two. one. none. Tags: Question 9 . SURVEY . 120 seconds . Q. Which of the following intermolecular forces plays a pivotal role in biological molecules such as proteins and DNA?

Ethene Molecule C2h4 Lewis Dot Cross Electronic Diagram Covalent Bonds Ball Stick Space Filling 3d Models Boiling Point Melting Point Doc Brown S Chemistry Revision Notes








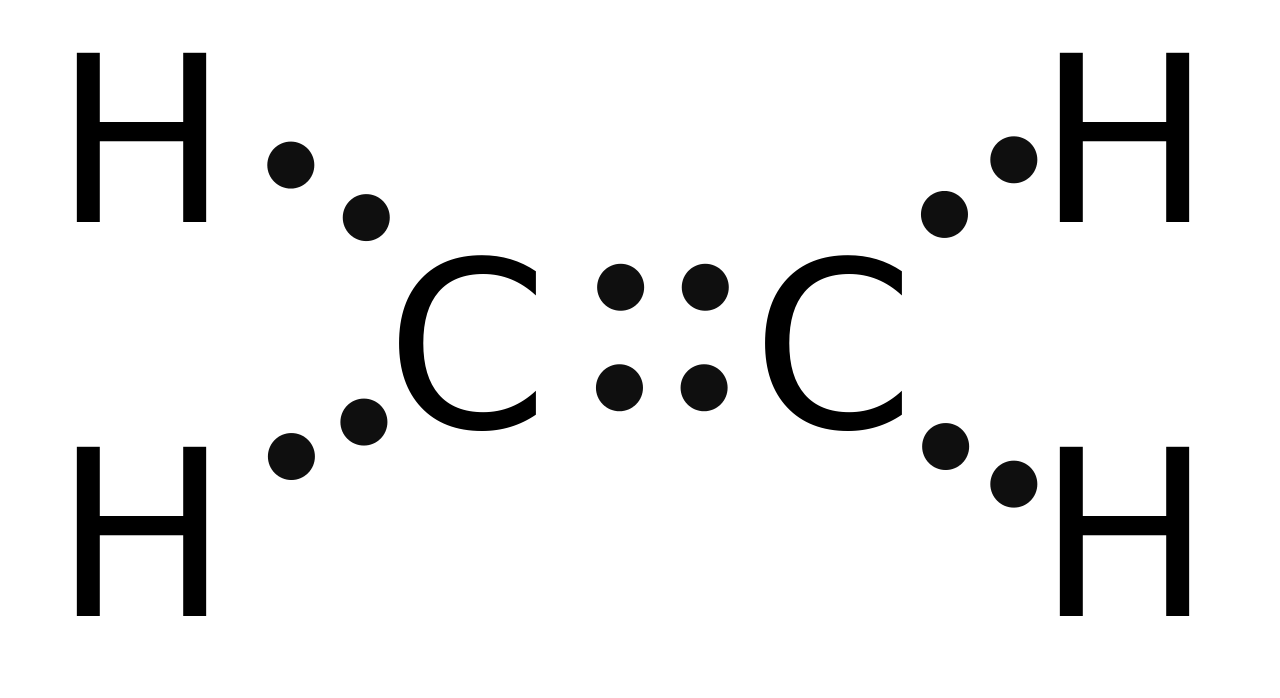



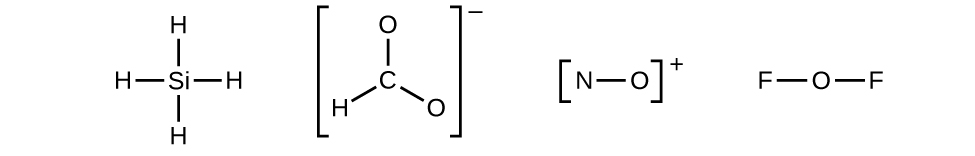





0 Response to "37 in an electron dot diagram of ethylene how many double bonds are present"
Post a Comment