38 solid liquid phase diagram
what is the shape of the phase diagram of a liquid mixture containing two compounds forming AB and A2B structural solids/lattice rather than forming A and B solids/lattice when melted? Forward: Almost seven months ago, I posted a two part story to this sub titled “Apheraitors.” It caught a modest number of eyeballs, but those who read it seemed to react surprisingly positively to it. To be honest, I was flattered. And, high on the rush of having pleased and impressed so many strangers on the internet, I promised more to come. And truth be told I had more. This story was already half written and a few others were outlined when I made that promise. But, I faltered. Part of ...
My house is neither old nor gothic, and at face value, there is absolutely nothing creepy about it. It sits on an expansive lot just far enough from town to feel pleasantly rural. The walls inside range from a crisp, clean white in the living room and breakfast nook to a cheery buttercup yellow in the hallways, kitchen, and office. The larger bedroom is a sweet lavender, while the smaller is the kind of little-boy blue that would’ve given away instantly that it had belonged to the former owner’s...
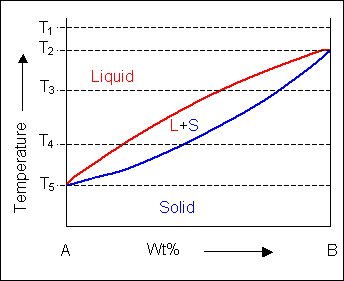
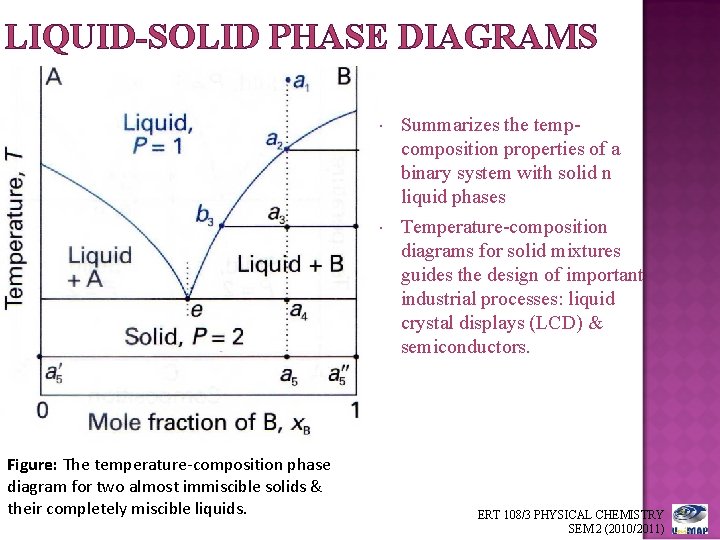
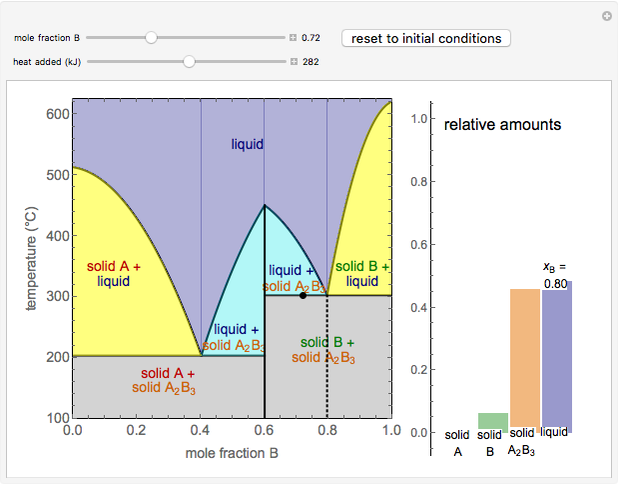
Solid liquid phase diagram
I'm trying to wrap my head around how ice can evaporate from getting even colder? Like in a pressurized freeze drier for example. I assumed liquids evaporated due to the loosened structure of molecules. Peritectic point - The point on a phase diagram where a reaction takes place between a previously precipitated phase and the liquid to produce a new solid phase. When this point is reached, the temperature must remain constant until the reaction has run to completion. This is the 874th online community I've tried. Hopefully, this will be the last. Terry told me about this place, told me you guys have experience with… weird stuff. The Chewy-Man isn't the only weird thing out there I'm sure. I know from the Bigfoot and UFO forums that every inexplicable or paranormal phenomenon has its storm chasers. I've got my fingers crossed that here this post finds someone, anyone, who might be able to shed some light on just what the heck is going on in my small town. ...
Solid liquid phase diagram. That being 25% NaCl, 25% KCl, 25% NaNO3, 25% KNO3 I find it very interesting to think about what dissolving actually does to the atoms involved, and especially what happens when things un-dissolve. I would assume that the ions would have no reason to reconnect with the things they were originally paired with, but I might get surprised! I understand that congruent melting means that the solid and liquid phases have the same composition, and that in incongruent melting, the third intermediate compound decomposes upon melting. However, I am having trouble relating this to the phase diagrams that supposedly depict congruent and incongruent melting. For [congruent](https://slideplayer.com/slide/13310126/80/images/14/Compounds+formation+with+congruent+melting+point..jpg) melting: does the liquid phase then contain all three compoun... Liquid, in physics, one of the three principal states of matter, intermediate between gas and crystalline solid. The most obvious physical properties of a liquid are its retention of volume and its conformation to the shape of its container. Learn more about the properties and behavior of liquids in this article. Jan 24, 2019 · Phase separation should be avoided during the purification process if the protein tends to undergo liquid-to-solid transitions. However, if dense phases can be disassembled without loss of material, several cycles of phase separation and dilution can be used as a purification method ( Quiroz and Chilkoti, 2015 ).
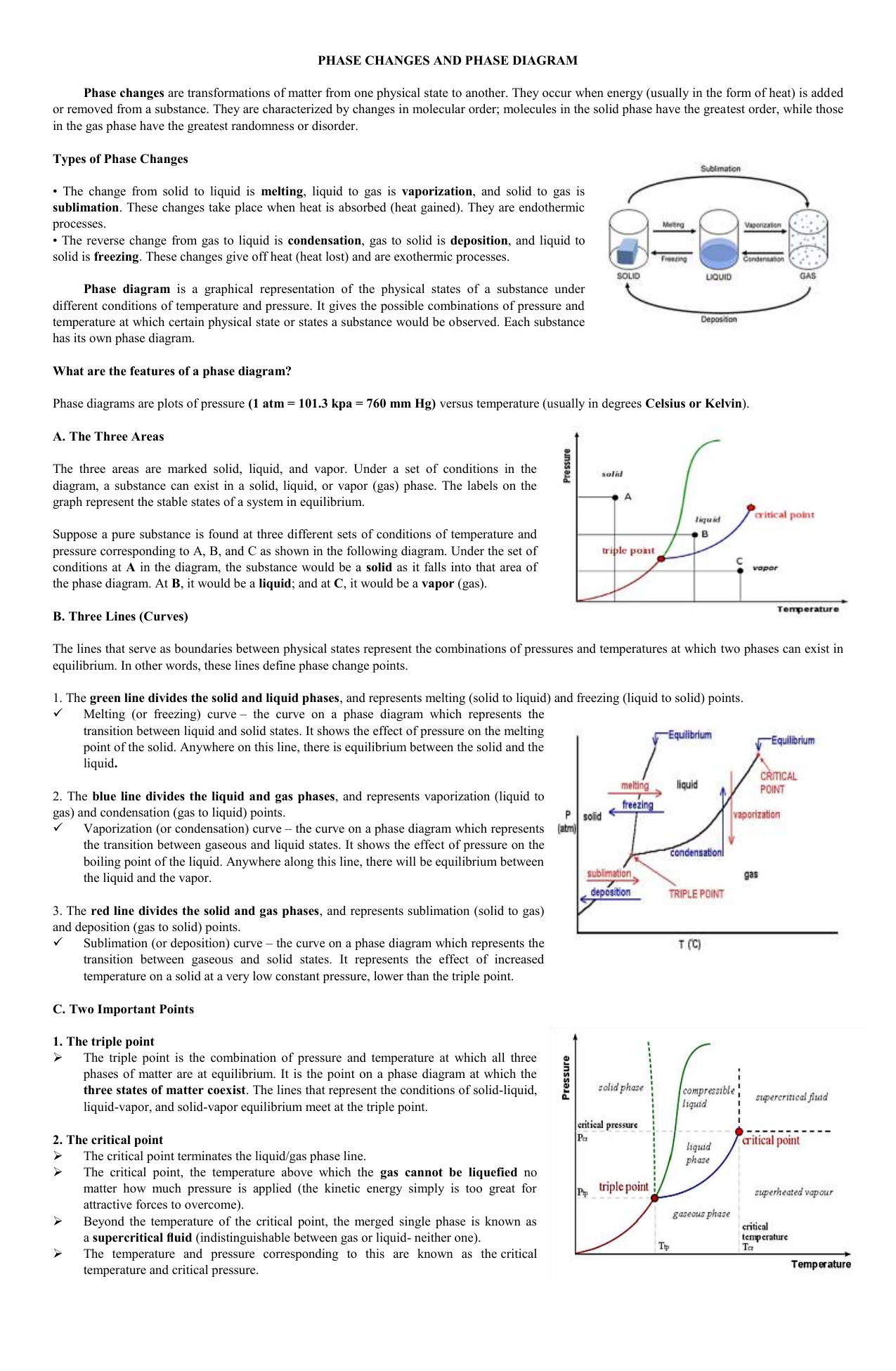
Hi, I was wondering if anyone would be able to direct me to some research papers/reputable data sources regarding the melting/boiling points of common hydrocarbons with respect to pressure. I have spent several hours searching via various search engines (both academic and otherwise) for data in academic journals, or simply solid-liquid phase diagrams for specific hydrocarbons, however I have been unable to find a verifiable source. The best I have found so far was a diagram for butane on Engine... Pretty straightforward. I've tried to look it up and I've asked some biology teachers I've had in previous years, but none of them have given me an actual answer besides "Triple point". Obviously the water can't be ice because ice is cold. Is hard warm water not possible? My daughter got one of those sodium acetate heating pads to warm her hands while ice skating. I found that sodium acetate can do this because it is easy to supercool it below the freezing point of 54C, so easy that just letting the sollution rest and cool down to far below it is possible. Then when you click the metal disk, it creates a staring point for the crystals to form. Aside from that I cant find any more detailed explanation, like what does the metal disk do that starts the reaction? ... Point B in this phase diagram represents the only combination of temperature and pressure at which a pure substance can exist simultaneously as a solid, a liquid, and a gas. It is therefore called the triple point of the substance, and it represents the only point in the phase diagram in which all three states are in equilibrium.
As per the notice, a suitable vehicle arrives just a few minutes late. We all pile into the machine and head towards the mine’s main portal. I figure we might just make this a driving tour, but find out, after consulting the maps, that it’s a one-way out of the mine with Land Cruiser-sized vehicles. However, once we get to the mine, there’s internal transport, so I’ve got that going for us, which is nice. I see that this old coal hole has 11 levels. Gad. I reel just thinking how much coal ha... This is the 874th online community I've tried. Hopefully, this will be the last. Terry told me about this place, told me you guys have experience with… weird stuff. The Chewy-Man isn't the only weird thing out there I'm sure. I know from the Bigfoot and UFO forums that every inexplicable or paranormal phenomenon has its storm chasers. I've got my fingers crossed that here this post finds someone, anyone, who might be able to shed some light on just what the heck is going on in my small town. ... Peritectic point - The point on a phase diagram where a reaction takes place between a previously precipitated phase and the liquid to produce a new solid phase. When this point is reached, the temperature must remain constant until the reaction has run to completion. I'm trying to wrap my head around how ice can evaporate from getting even colder? Like in a pressurized freeze drier for example. I assumed liquids evaporated due to the loosened structure of molecules.

Solid Liquid Phase Diagram Of The Binary System Octadecanoic Acid And Octadecanol And The Thermal Chemical Property Of The Composition At Eutectic Point

Use The Diagram Provided To Answer Each Of He Following Questions A Which Section Represent The Liquid Phase B Which Section Represent The Solid Phase C Which Section Represent The Gas Phase
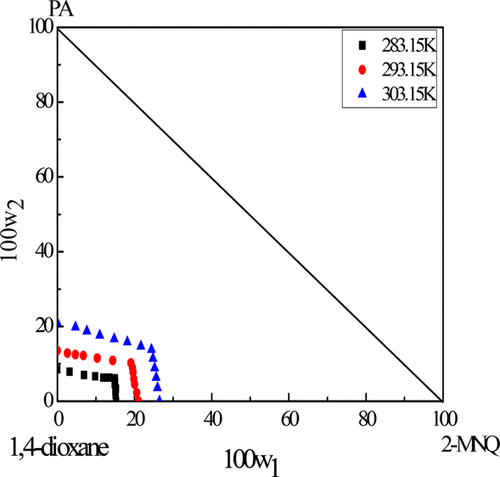
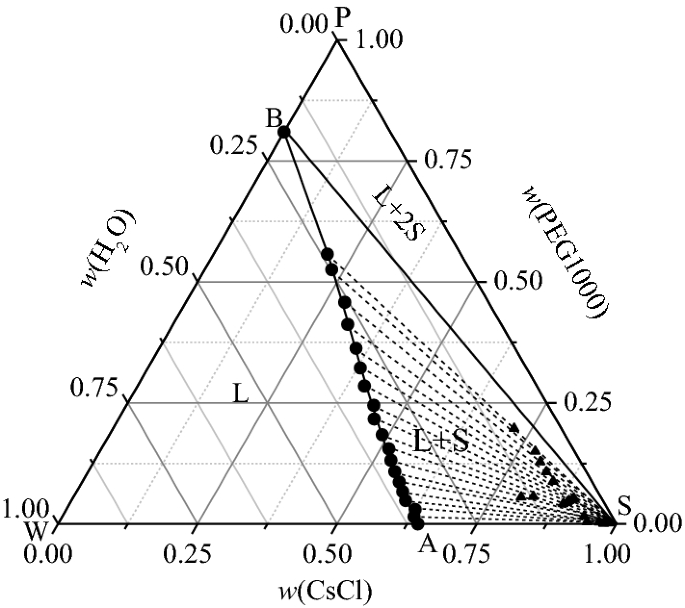
Solid Liquid Equilibrium And Phase Diagram For Ternary 2 Methyl 1 4 Naphthoquinone Phthalic Anhydride 1 4 Dioxane System Journal Of Chemical Engineering Data X Mol


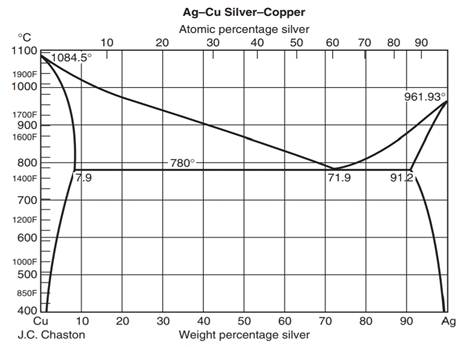


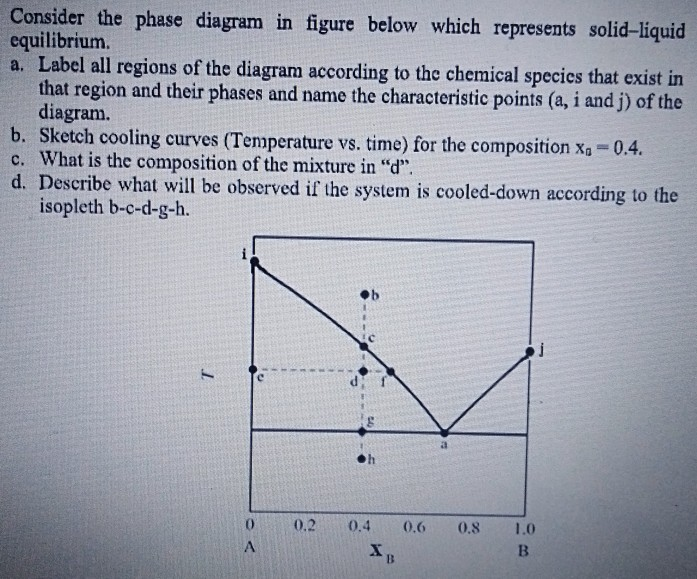












0 Response to "38 solid liquid phase diagram"
Post a Comment