39 cobalt electron dot diagram
Lewis-Dot Diagrams •Lewis Dot Diagrams are a way to represent the valence electrons in an atom. –Element’s symbol represents the nucleus and inner-level electrons –Dots represent the valence electrons 5.3 Electron Configuration. Lewis-Dot Diagrams •Dots are placed one at a time on the four Now the Oxygen has 8, but the Carbon has 8 as well. So by now we've used all the valence electrons, all 10, and each of the atoms in the Lewis structure for CO has a full outer shell--has an octet, with 8 valence electrons. So that's the Lewis structure for CO, carbon monoxide. This is Dr. B., and thanks for watching.
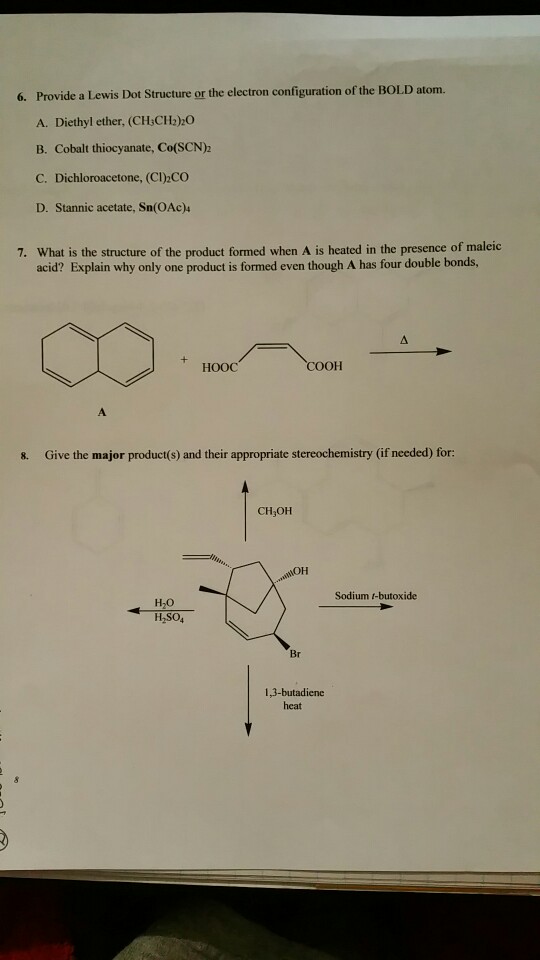
An electron dot structure is a diagram that shows the symbol of an element and its valence electrons as dots Metallic atoms tend to ____________ valence electrons to produce a positively charged ions.

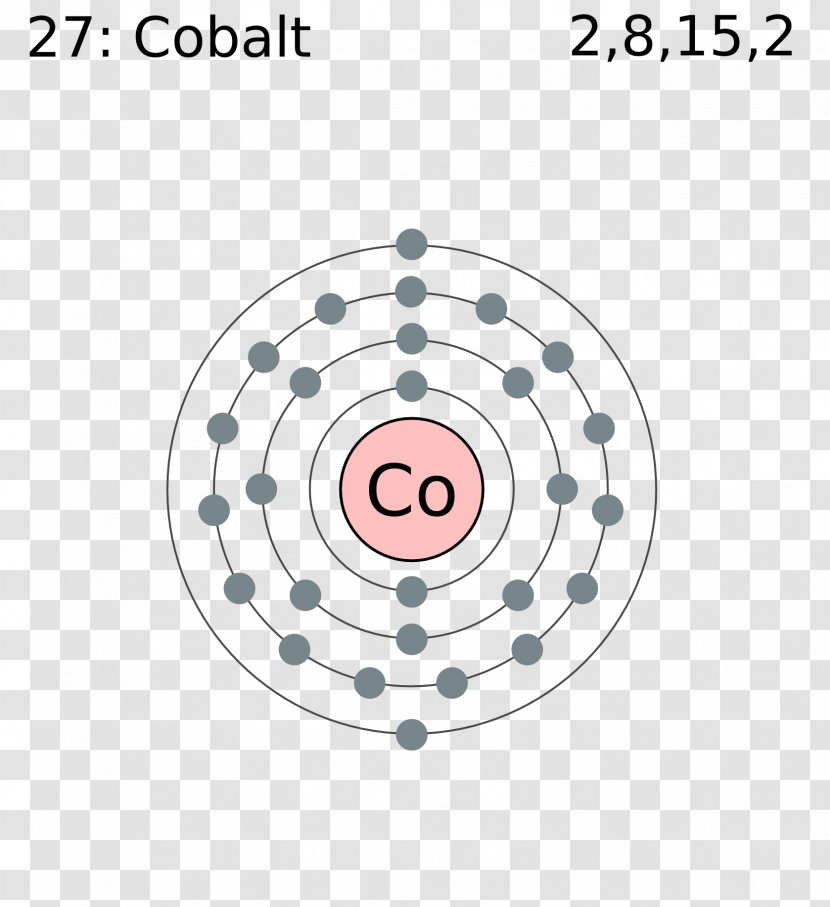
Cobalt electron dot diagram
An electron-dot structure (Lewis structure) of NO 2 - is shown below. Indicate which is a correct resonance structure for NO 2 -. Structure #1, Structure #2, Structure #3, and Structure #4 are all incorrect resonance structures for NO2-. Answer to: Draw and explain the orbital diagram for cobalt (Z = 27). ... how to draw Lewis dot structures and see resonance in Lewis dot structures using the benzene Lewis dot structure example. Cobalt sulfate is an odorless rose-pink solid. Sinks and mixes with water. (USCG, 1999) Cobaltous Sulfate is a reddish, toxic, metallic salt. Cobalt sulfate is used in the electrochemical industries, as a drier in paints and inks, as a coloring agent, in storage batteries and as a supplement for Vitamin B12 deficiency.
Cobalt electron dot diagram. The Lewis structure for CO has 10 valence electrons. For the CO Lewis structure you'll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule. YouTube. Wayne Breslyn. 407K subscribers. Name: Cobalt Symbol: Co Atomic Number: 27 Atomic Mass: 58.9332 amu Melting Point: 1495.0 °C (1768.15 K, 2723.0 °F) Boiling Point: 2870.0 °C (3143.15 K, 5198.0 °F) Number of Protons/Electrons: 27 Number of Neutrons: 32 Classification: Transition Metal Crystal Structure: Hexagonal Density @ 293 K: 8.9 g/cm 3 Color: silver Atomic Structure In order to write the electron configuration we first need to know the number of electrons for the Cobalt (Co) atom. There are 27 electrons for the Cobalt a... Oct 04, 2009 · the Lewis dot diagram is a table used for the elements, and it shows you how many valence electrons there are. What is the Lewis dot diagram for Mercury? No no no Hg is Mercurys Atomic symbol.
Lewis Dot Diagrams of Selected Elements. Lewis Symbols: Electron Configuration into Shells: Index Chemical concepts Chemistry of the Elements Periodic Table . HyperPhysics***** Quantum Physics : R Nave: Go Back: Electron Distributions Into Shells for the First Three Periods. A chemical element is identified by the number of protons in its ... The electron dot diagram for an element shows the valence electrons for the element. Oxygen is in group 16/VIA, so it has six valence electrons. Draw the symbol for oxygen. Then place one dot at each side of the symbol. There are now four unpaired electrons around the oxygen symbol. Electron Configurations. The content that follows is the substance of General Chemistry Lecture 26. In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements. Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
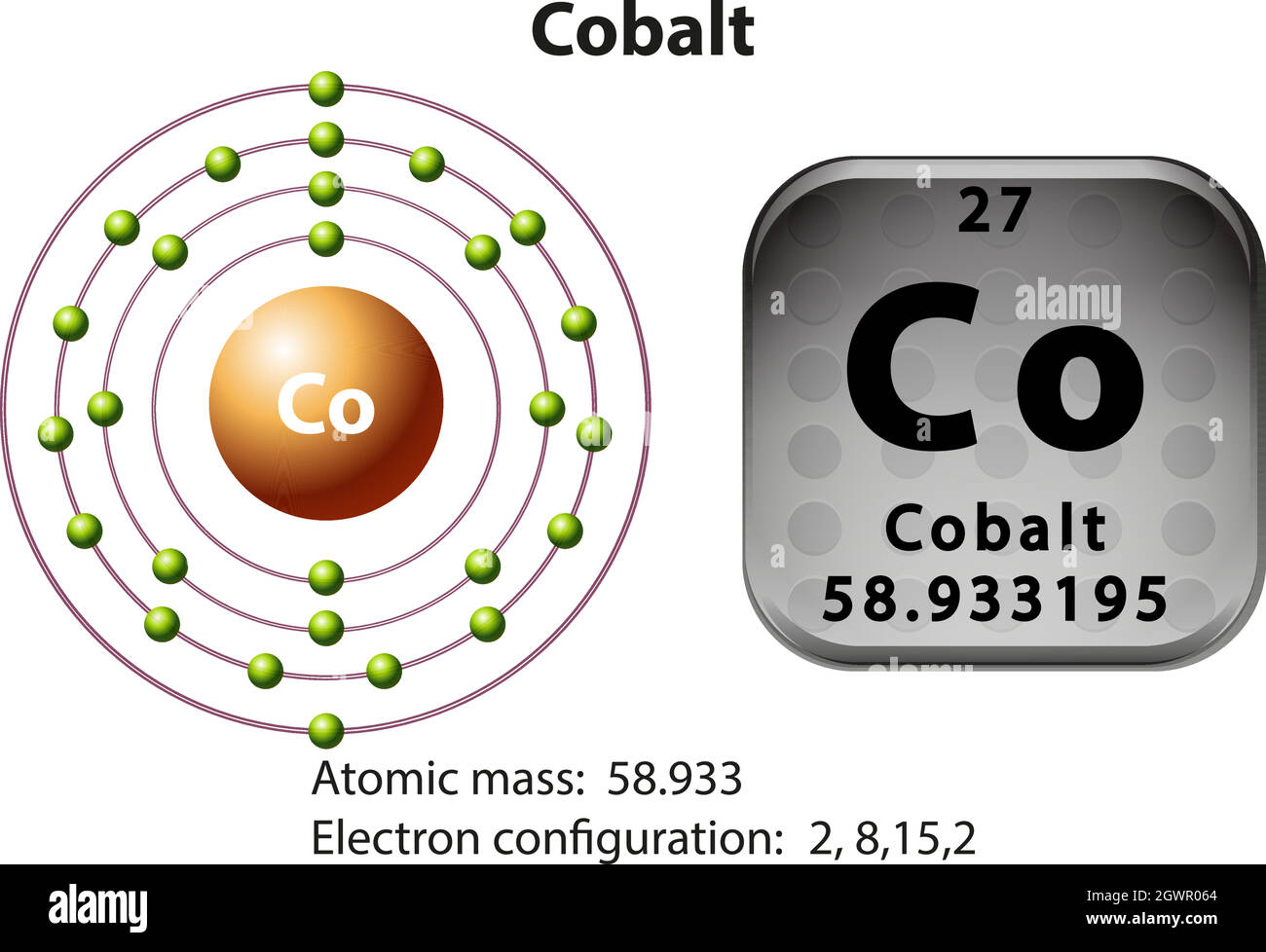
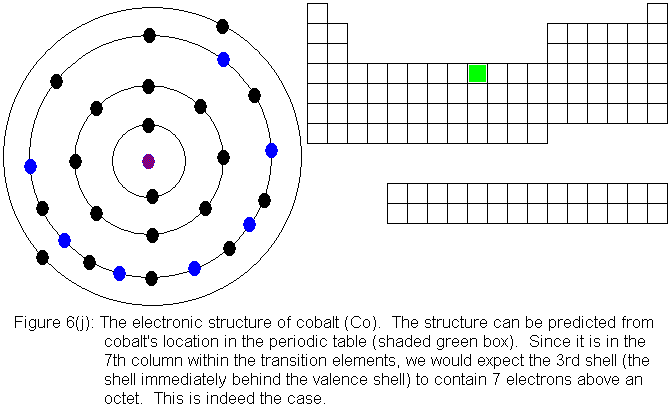
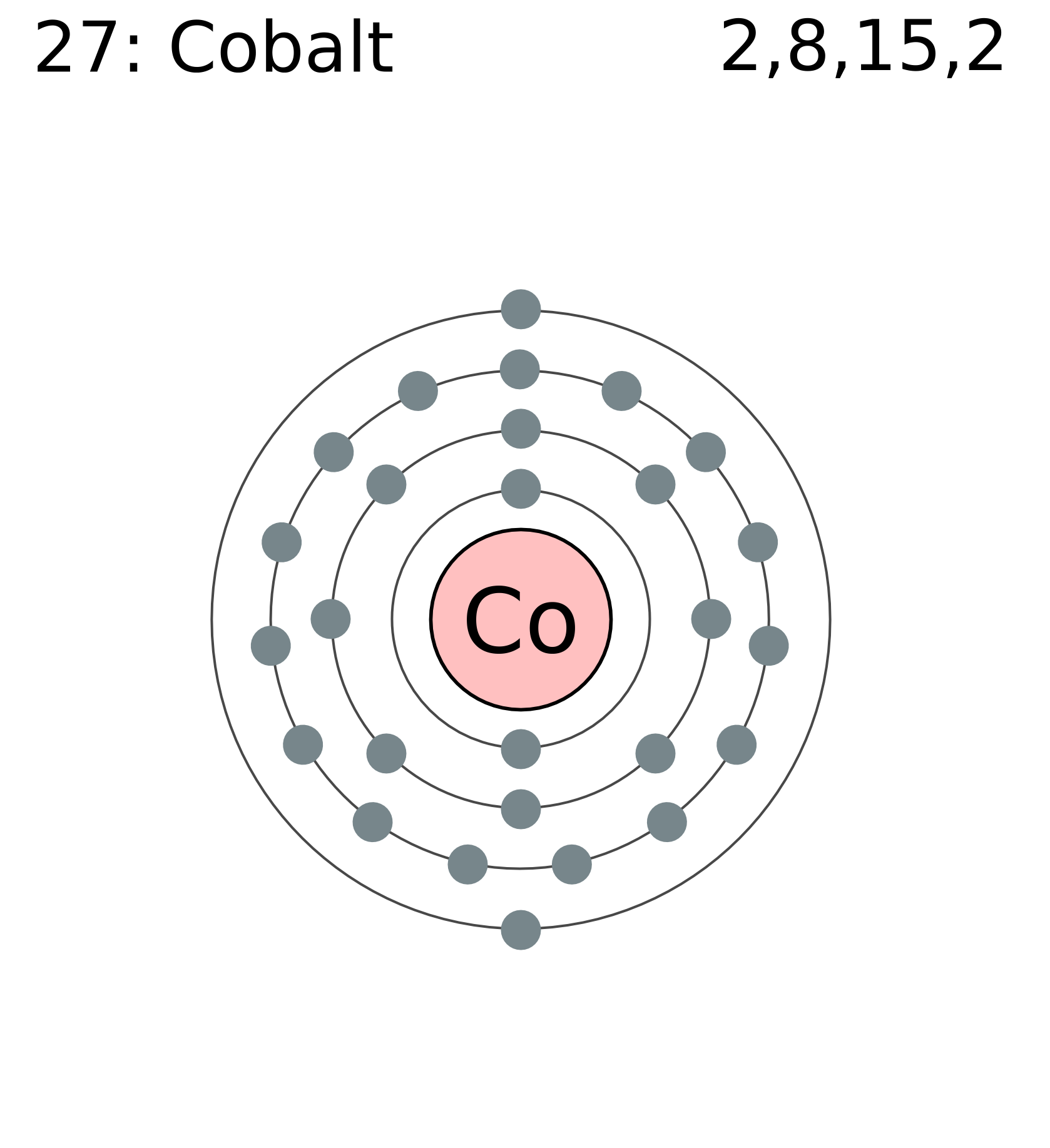
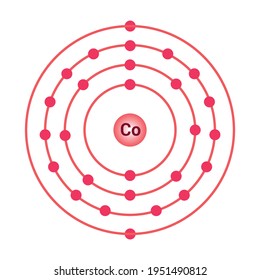
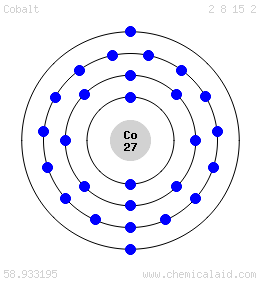
3. Electron Dot shows only the valence (outer energy level) electrons. . Ex. Oxygen atom . O :. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams . for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Figure: the electron configurations of cobalt and bromine. The Valence electrons are underlined in red. Electron Dot Diagrams . The valence electrons of an atom are the electrons located in the s and p sub-level of the highest energy level. Valence electrons are primarily responsible for the chemical properties of elements. The Lewis dot diagram for Platinum is a diagram showing bonds & electrons of the Platinum atom within a molecule. Nobody will be able to draw you a diagram here, as this is a text-only answer board. Use Google Image Search if you want to actually see the Lewis diagram for Pt.Cobalt Bohr ModelLewis dot diagram for cobalt. Answer (1 of 6): Cobalt (Co) has an atomic number of 27, which means it has 27 electrons and protons. Shells hold up to two electrons in the first and eight electrons in the outer shells. So cobalt's electron configuration with 27 electrons should look like this: 2-8-8-8-1. However, each shell i...
The Lewis Dot Structure is a graphical representation of how electrons are distributed around the atoms which comprise a molecule. The reason for drawing/creating a Lewis dot structure is that it helps one predict the kinds of bonds, as well as a number of bonds, that can be formed around an atom. Lewis structures can be utilized to make ...
It is K with one dot so: K . The reasoning behind this is that you put the highest energy level on the dot notation. Electron Configuration notation for Potassium is: 1s2; 2s2, 2p6; 3s2, 3p6, 4s1.
The lewis dot structure is a diagram to show the bonding between the atoms of a molecule and pairs of electrons that may exist. This eight electrons are found in four pairs. I show you where magnesium is on the periodic table and how to determine how many valence electrons magnesium has. Ionic bonding chemistry for non majors.

Today Is Thursday October 16 Th 2014 Pre Class When We Were Naming Things Like Co 2 And Hno 3 And Srf 2 What Were All The Atoms In Those Compounds Ppt Download
1 point is earned for a correct Lewis diagram. (ii) In Box Y below, draw the complete Lewis electron-dot diagram for the other compound, which is a structural isomer of the compound represented in Box X. Include any lone (nonbonding) pairs of electrons. 1 point is earned for a correct Lewis diagram.
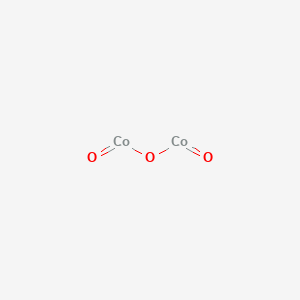
A step-by-step explanation of how to draw the Co(NO3)2 Lewis Dot Structure.For Co(NO3)2 we have an ionic compound and we need to take that into account when ...
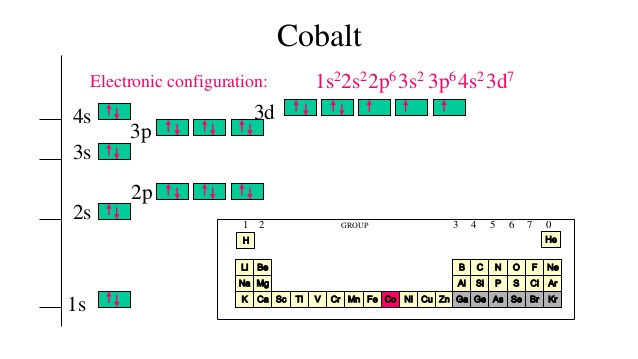
The electron configuration for cobalt is 1s2 2s2 2p6 3s2 3p6 3d7 4s2. The first number in each group identifies the energy level of the electrons. The letter represents the type of shell in which the electrons sit, while the final number denotes the number of electrons in the shell. The electron configuration for cobalt can be shortened to [Ar ...
Lewis Structure (electron dot diagram) for the oxygen molecule, O 2, OR . There are 2 bonding pairs of electrons shared between the 2 oxygen atoms, and each oxygen atom also has 2 lone pairs (non-bonding) pairs of electrons. In the Valence structure for the oxygen molecule, each bonding pair of electrons is replaced by a dash (-) to represent a ...
Cobalt (Co) has an atomic mass of 27. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. ... Electron Configuration [Ar] 3d 7 4s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 7 4s 2: Orbital Diagram. 1s ...
Oct 22, 1995 · Uses of Cobalt: Used in many hard alloys; for magnets, ceramics and special glasses. Also used in permanent magnets, razor blades and catalitic converters. Cobalt-60 is used in cancer therapy. Additional Notes: Cobalt Menu. Cobalt Page One. Overview of Cobalt; Cobalt's Name in Other Languages; Atomic Structure of Cobalt; Chemical Properties of ...
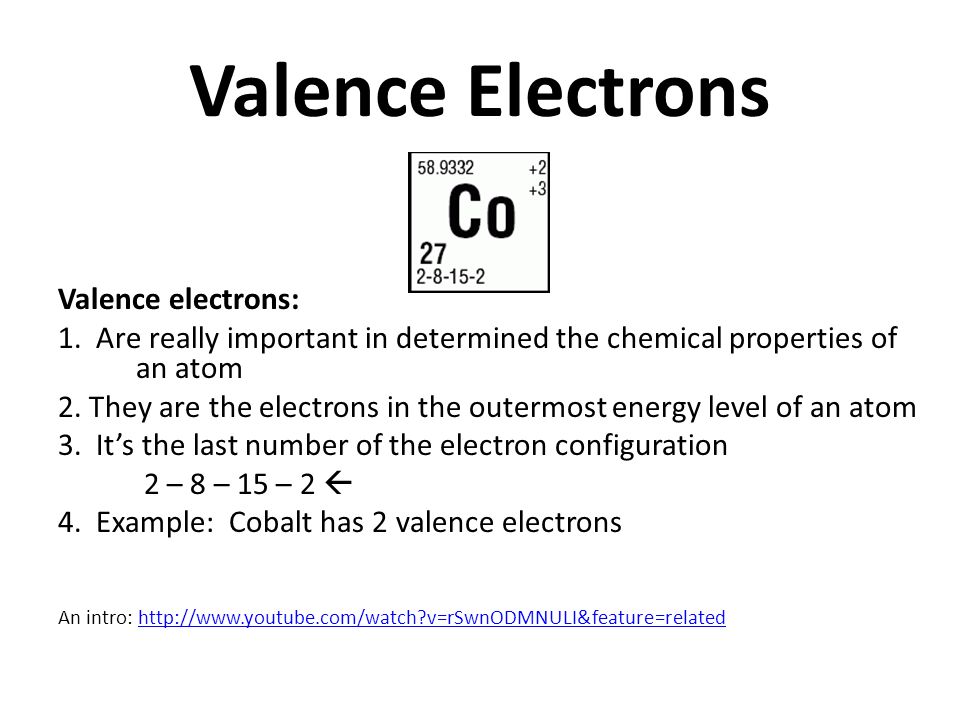
A Lewis Dot Structure is a way of showing the valance electrons in an element. Cobalt has two so there are two dots. Orbital Diagram of Cobalt
A cobalt salt in which the cobalt metal is in the +2 oxidation state and the counter-anion is chloride. It is used as an indicator for water in desiccants. ChEBI CHEBI:35696. Predicted data is generated using the ACD/Labs Percepta Platform - PhysChem Module. Density:
Correlation One On Twitter Ds4a Empowerment Cohort 3 Application Window Closes This Friday Gain A Competitive Edge In Today S Job Market With In Demand Skills And Apply Today Https T Co Hr7qmnxmu8 Dataliteracy Professionaldevelopment
Lewis-Dot Diagrams •Lewis Dot Diagrams are a way to represent the valence electrons in an atom. -Element's symbol represents the nucleus and inner-level electrons -Dots represent the valence electrons 5.3 Electron Configuration. Lewis-Dot Diagrams •Dots are placed one at a time on the four
Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is ...
The s,p,d,f configuration for cobalt (Co) is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^7#, determined by the position of the element on the periodic table.. Cobalt is an inner transition metal which means the electron configuration will end in a d block. Cobalt is in the 7th column of the d block and therefore has 7 d electrons #d^7#.The element cobalt can be found in the 4th row or 4th energy level ...
Electron Dot: b. Cobalt: Outer Shell Orbital Notation: Outer Shell Electron Configuration: Electron Dot: c. Antimony: Outer Shell Orbital Notation: Outer Shell Electron Configuration: Electron Dot: 2. For each of the following ions, show the orbital notation (regular), electron configuration (regular), and electron dot picture: a. Aluminum (+3 ...
The formal charges being 0 for all of the atoms in the CoCl 2 molecule tells us that the Lewis dot structure presented above is stable.. Thus, the Lewis structure of CoCl 2 is an exception to the octet rule.. Therefore, the Lewis Structure for the CoCl 2 is represented as follows:. CoCl2 Hybridization. To determine the hybridization of Cobalt Dichloride, we first determine the number of ...
Cobalt sulfate is an odorless rose-pink solid. Sinks and mixes with water. (USCG, 1999) Cobaltous Sulfate is a reddish, toxic, metallic salt. Cobalt sulfate is used in the electrochemical industries, as a drier in paints and inks, as a coloring agent, in storage batteries and as a supplement for Vitamin B12 deficiency.
Answer to: Draw and explain the orbital diagram for cobalt (Z = 27). ... how to draw Lewis dot structures and see resonance in Lewis dot structures using the benzene Lewis dot structure example.
An electron-dot structure (Lewis structure) of NO 2 - is shown below. Indicate which is a correct resonance structure for NO 2 -. Structure #1, Structure #2, Structure #3, and Structure #4 are all incorrect resonance structures for NO2-.

Warm Up Name The Following Compounds Cu 2 Sca No 3 2 2 Write The Chemical Formula For These Compounds Cobalt Iii Sulfide Zinc I Phosphate Ppt Download



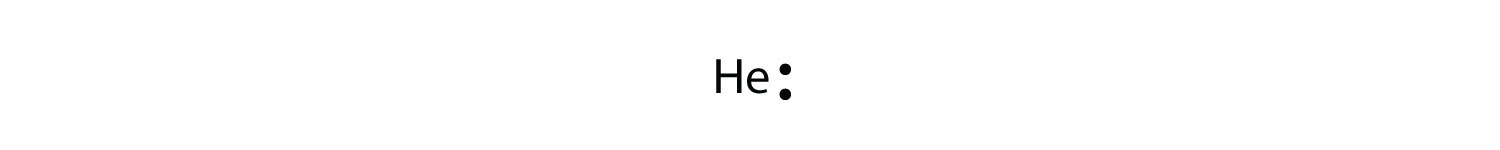













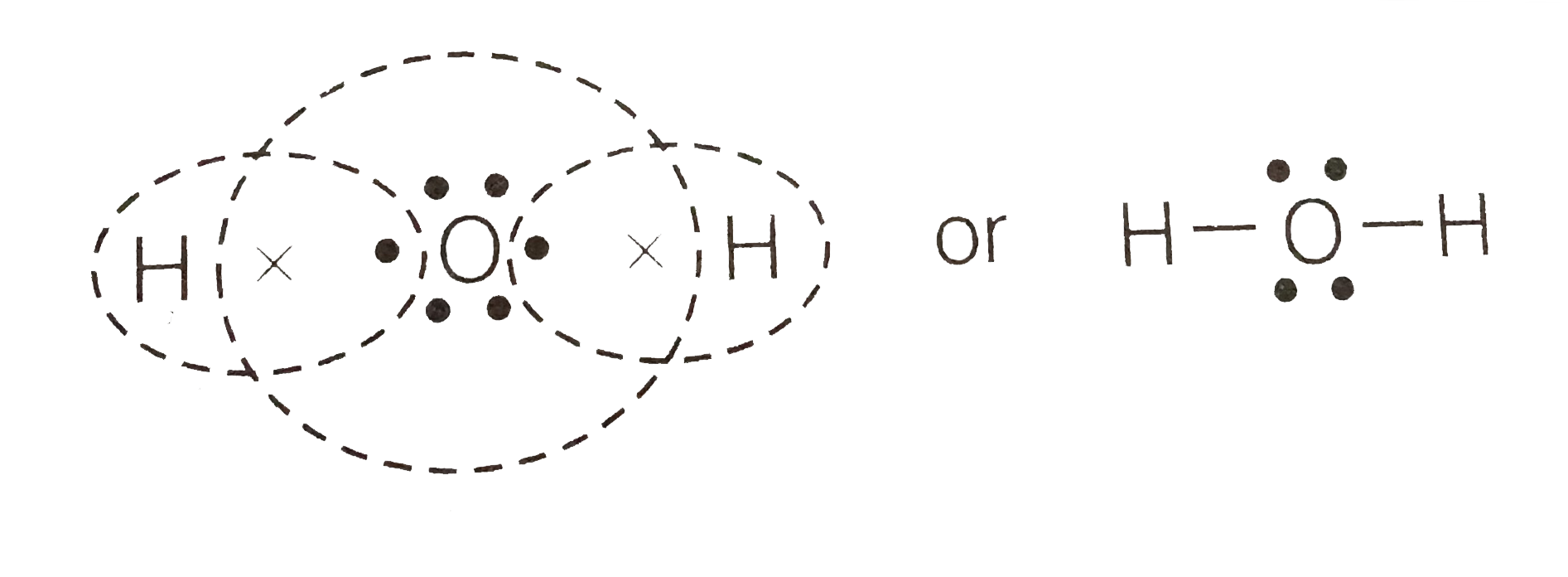

0 Response to "39 cobalt electron dot diagram"
Post a Comment