34 orbital diagram of fe
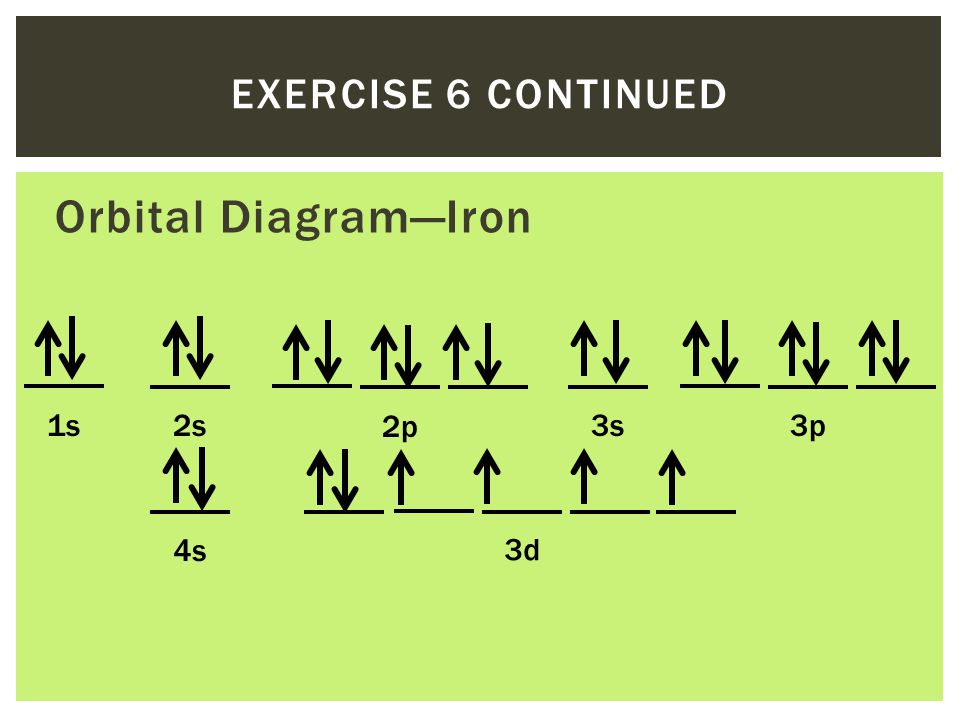
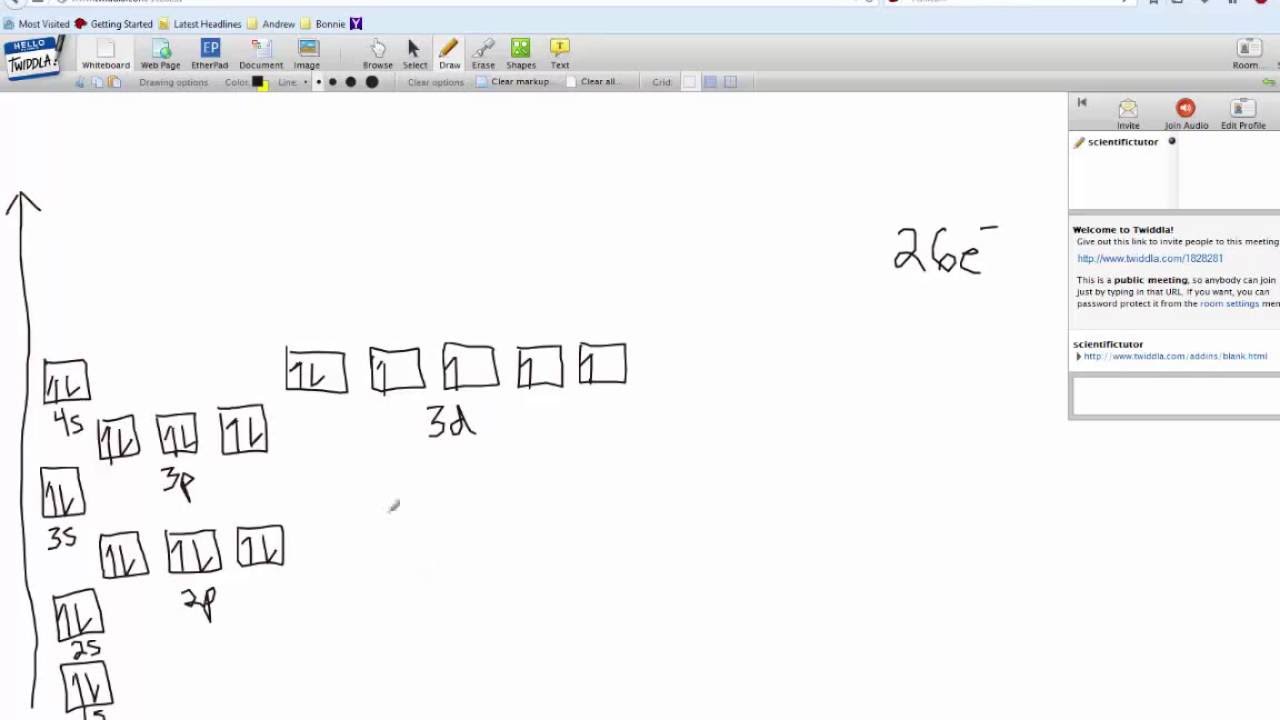
ORBITAL DIAGRAM Y ↿⇂ 1s ↿⇂ 2s ↿ ↿ ↿ 2p N Fill orbitals with "up" before "down" to maximize number of unpaired electrons. ↿⇂ 1s ↿⇂ 2s ↿⇂ ↿⇂ ↿⇂ 2p Na ↿⇂ 3s ↿⇂ 3s Fe ↿⇂ ↿⇂ ↿⇂ 3p ↿⇂ 4s ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 3d Let's only worry about the outermost orbitals for Fe.
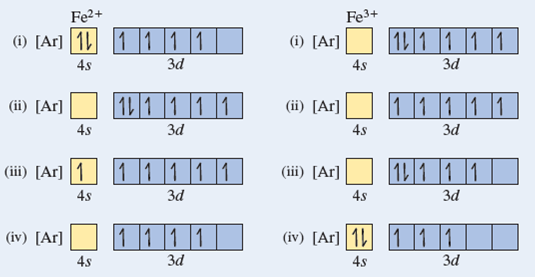
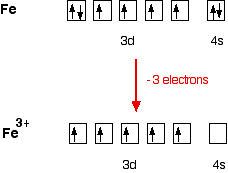
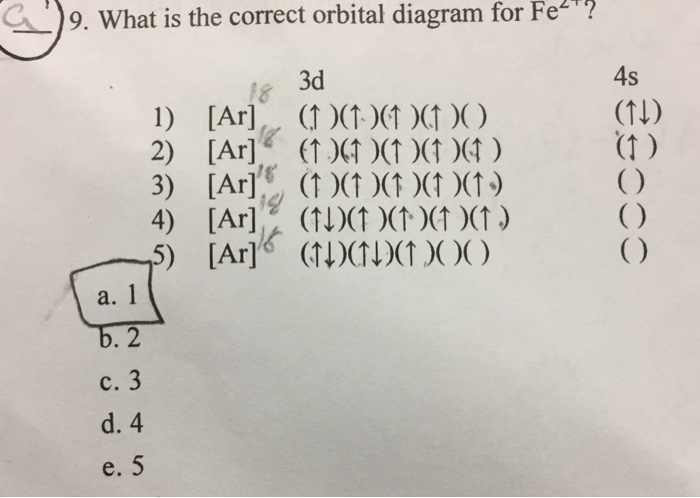
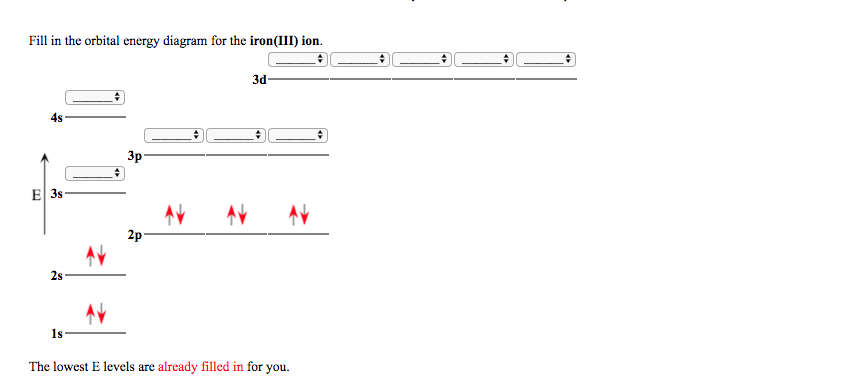
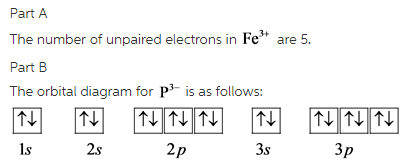
Orbital Diagram For Fe3+. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). Fe3+ loses an electron from the d You take electrons out of the 4s orbital, before you take them out of the 3d orbital, because it is lower. -3,-2,-1,0,1,2,3 c.
Iron (Fe) has an atomic mass of 26. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.

Orbital diagram of fe
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.electron configuration for Fe2+ - CHEMISTRY COMMUNITYMolecular orbital diagram - Wikipedia
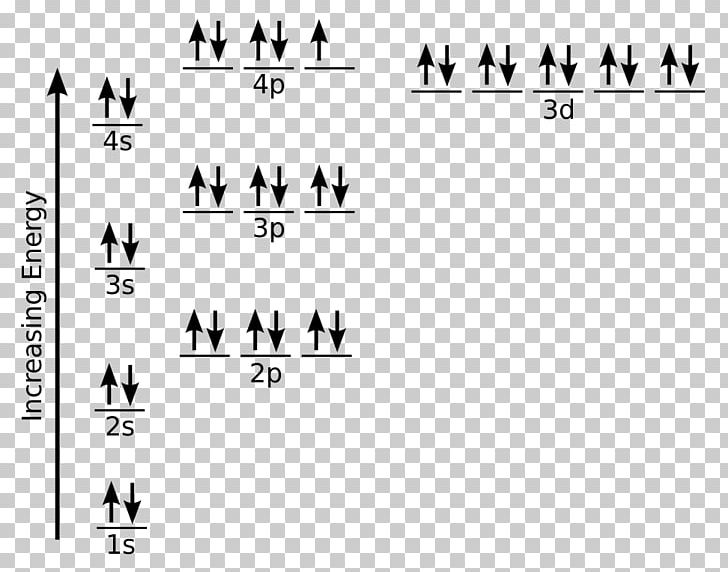
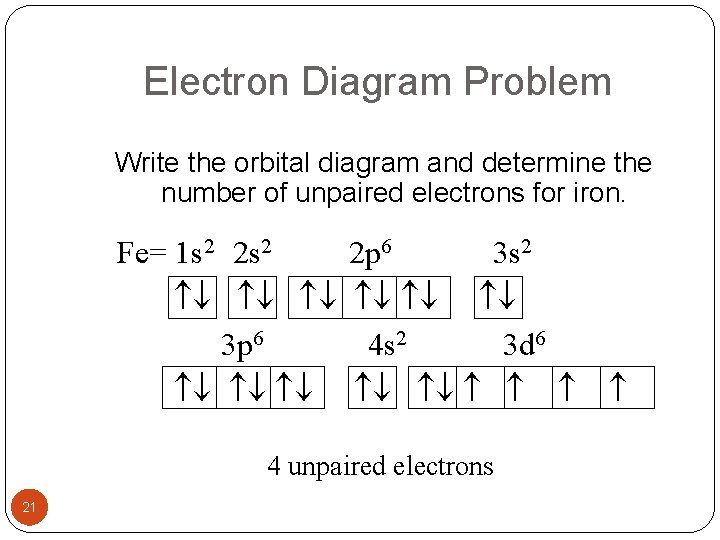
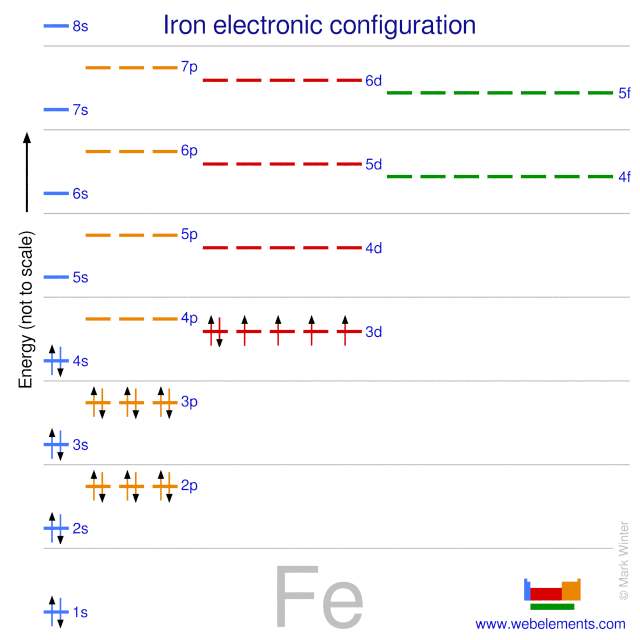
Fe or iron has the atomic number of 26. Its full orbital diagram is 1s2 2s2 2p6 3s2 3p6 4s2 3d6. We have chosen to show the full unabbreviated configurations to provide more practice for students who want it but listing the core abbreviated electron configurations is also.
Download scientific diagram | Qualitative valence molecular-orbital (MO) diagram of Fe(CO) 5 . Displayed is the subset of Fe(CO) 5 MOs which are derived from Fe 3d and CO 5r and 2p orbitals. For ...
Orbital diagram of fe.
Electron Configurations. The content that follows is the substance of General Chemistry Lecture 26. In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements.
Iron (Fe) is a transition metal that follows the Aufbau rule of the filling of atomic orbitals. The atomic number of Fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron clouds. The ground state electron configuration of Fe is: 1s22s22p63s23p63d64s2
How many d electrons are in FE? From the periodic table, iron has atomic number 26 , meaning that there are 26 electrons in each ground state iron atom. 2⋅5=10 electron in each d orbital, and so on so forth. Electron orbitals fill according to the Aufbau (Build-up) Principle. Why Fe2+ is easily oxidized to Fe3+?
Fe orbital Diagram. what is the orbital diagram for fe answers fe or iron has the atomic number of 26 its full orbital diagramis 1s2 2s2 2p6 3s2 3p6 4s2 3d6 electron configuration orbital diagram iron electron configuration orbital diagram iron how to write electron configurations and orbital diagrams duration fe fe2 & fe3
Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine ...
As is apparent from the molecular orbital diagram of excited Fe(CO) 4 in Fig. 2b, these transitions entail negative energy transfer because the incident photon energy is smaller than the scattered ...
r====- Fe(t,- ) Fe(t2s ) Fig.2. Molecular orbital diagram for the Fe3*Mn2*O,o clus-ter in the (a) ferromagnetic and (b) antiferromagnetic configu-rations. Orbitals indicated with a dashed line are unoccupied. Note that the orbital energies correspond to "orbital electronega-tivities" (Slater, 1974). The energy differences between orbitals
What is the orbital diagram for Fe? - Answers Fe, or iron, has the atomic number of 26. diagram is 1s2 2s2 2p6 3s2 3p6 4s2 3d6. Home Study Guides Science Math and Arithmetic History Literature and...
After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d6. Therefore the Iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Note that when writing the electron configuration for an atom like Fe, the 3d is usually written before the 4s. Both of the configurations have the correct numbers of ...
• The primary orbital interactions that form the metal‐ligand bonds in ferrocene occur between the Fed orbitalsand the ‐orbitals of the Cp ligand. • If D 5d symmetry is assumed,so that there is a centre of symmetry in the ferrocene molecule through the Fe atom there will be centro ‐symmetric (g)andanti‐ symmetric(u) combinations.
d-orbital diagram for [Fe(H 2 O) 6] 3+: The first three electrons go into t 2g orbitals unpaired. The 4th and 5th electrons must choose whether to pair up with electrons already in t 2g (which costs energy) or to go into higher energy e g orbitals (which also costs energy). In this case, the splitting energy is less than the pairing energy so the 4th and 5th electrons go into the e g orbitals.
The complex ion [Fe (CN)6]3- has an octahedral geometry owing to the six cyano ligands coordinated to Fe. Since CN- is a strong field ligand, there is pairing of electrons on the sub orbitals of 3d orbital hence the hybridization expected on the Fe3+ is d2sp3 (a low spin inner orbital complex ion). 1.6K views Sponsored by Best Gadget Advice
c) draw the energy diagram of d-orbital splitting for [Fe(CN)6] 3- and place the electrons on it. CN- is a strong field ligand. d) how many unpaired electrons are there in [Fe(CN)6] 3- ? e) now consider the complex ion [FeCl6] 3- , in which Clis a weak field ligand.
Fe:[Ar]3d⁵ . How many valence electrons does an arsenic atom have? ... Which principle or rule is violated by the following orbital diagram of an atom in its ground state? ** the second 2p orbital is empty. Hund's rule. The element whose atoms in the ground state have 2 half-filled orbitals is. Po.
A schematic molecular orbital diagram of Fe (CO) 5 constructed from density-functional theory calculations. The isosurface plots of the occupied and unoccupied orbitals have been obtained with...
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
11.09.2018 11.09.2018 5 Comments on Fe2+ Orbital Diagram For midterm question Q5C, why the electron configuration for Fe2+ is not [Ar]3d^5 4S^1? The outermost shell, in this case, is the 4s orbital.
Write the complete orbital configuration for iron (Fe). Orbital Configuration: Orbital configuration is the arrangement of electrons present in the atomic structure.
Example: Constructing MOs for Titanium Tetraiso propoxide, Ti(OiPr) pp , 4 • The OiiPrSALCs are compridised of fill dfilled p d bit l th O t x an p y orbitals on e a oms. • Ti bonding AOs E: (3dz 2, 3dx2‐y ) T 2: (4p x , 4p y, 4p z) (3dxy, 3dxz, 3dyz) • The T 1 SALC is non‐bonding. • Significant overlap occurs between the E SALC 2and the e AOs on Ti (3dz2, 3dx2‐y )
Orbital Notation for Iron (Fe). Mr. Causey shows you step by step how to write the orbital notation for iron (Fe).http://yourCHEMcoach.comSUBSCRIBE for more ...
2. Molecular orbital theory: This is the best model to explain the bonding within the CO ligand as well as in metal carbonyl complexes. There are total three molecular diagrams for carbonyl ligand which were proposed from time to time. Though, all three molecular orbital (MO) diagrams are able to explain the nature of metal-4
Fe3+ loses an electron from the d You take electrons out of the 4s orbital, before you take them out of the 3d orbital, because it is lower.An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represe nt the electrons in each orbital.
Re: Electron Configuration for Fe 3+. When I work this out, I first write out the structure for the ground state configuration which would be [Ar] 3d^6 4s^2 and then I just take electrons out in the order that is written so Fe3+ would be [Ar]3d^5 since the 4s block has slightly lower energy and they are filled first. Hope this helps!

Write the orbital diagram corresponding to the ground state of nb whose configuration is leftmathrmk


















0 Response to "34 orbital diagram of fe"
Post a Comment