35 vapor liquid equilibrium diagram
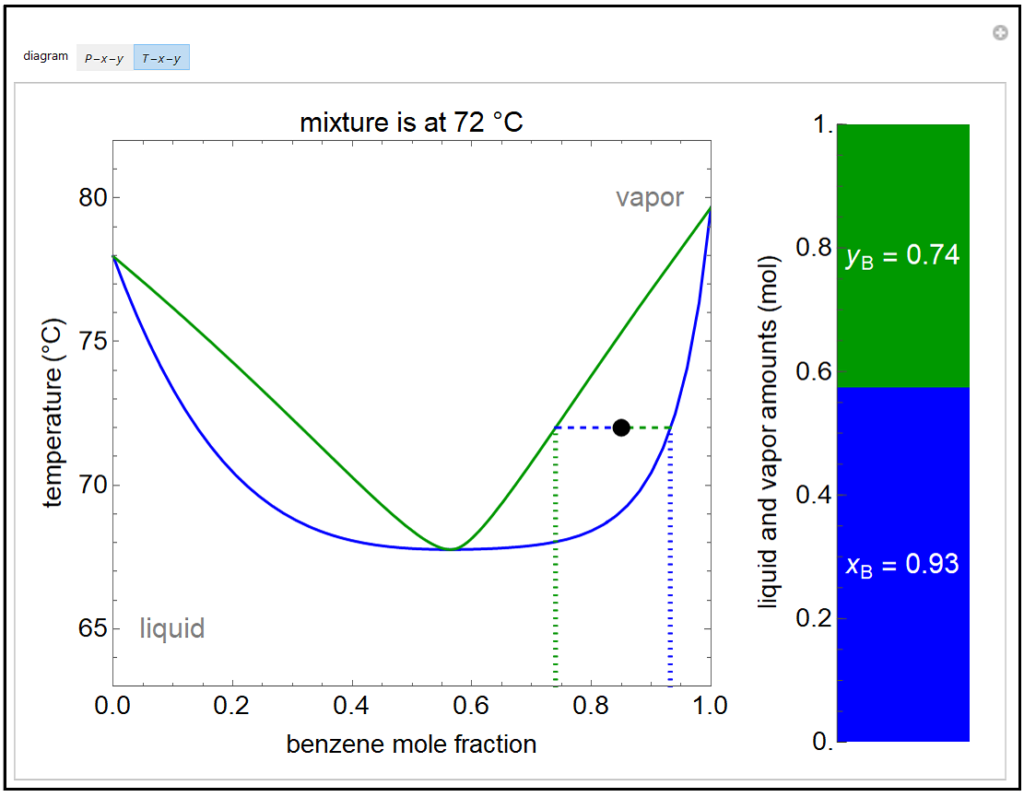
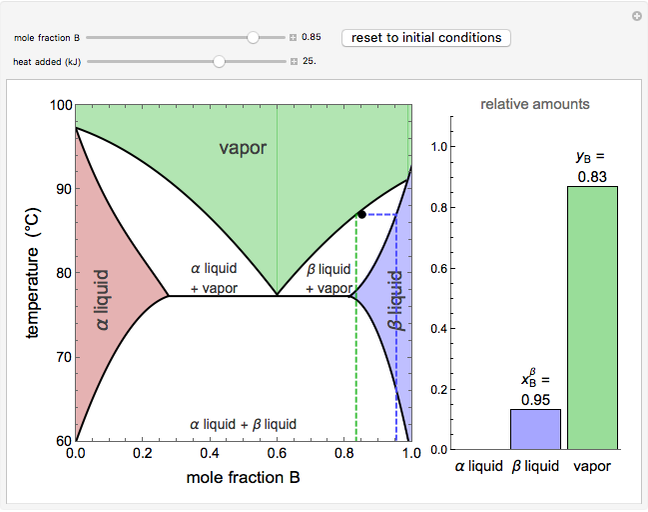
Because of the partial miscibility, vapor-liquid equilibrium (VLE), liquid-liquid equilibrium (LLE), and vapor-liquid-liquid equilibrium (VLLE) are present on the phase diagram. You can vary the mole fraction of component B and the heat added (location of the black dot).
The ability to accurately correlate vapor-liquid equilibria of pure substances is important, because systematic errors observed in such calculations will usually propagate into mixture calculations. Fig. 3 shows the vapor-liquid equilibrium of methane, propylene, diethylether and toluene in a T-ρ-diagram. The densities of the coexisting phases ...
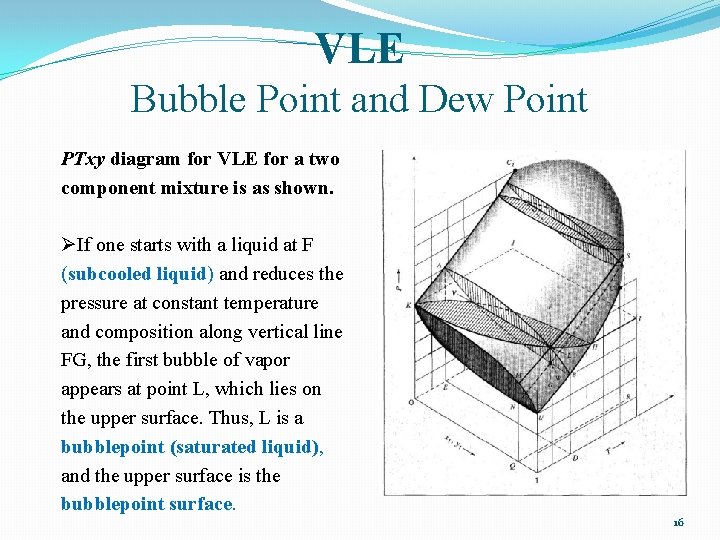
The combination of these diagrams lead to a 3-dimensional surface. Page 6. involving pressure, temperature and phase compositions on the three axes. A typical ...39 pages

Vapor liquid equilibrium diagram
Vapor Liquid Equilibrium Diagram at the Normal Boiling Point 0 10 20 30 40 50 60 70 80 90 100 0 1020 3040 50 6070 8090 100 Weight Percent HF in Liquid Weight Percent HF in Vapor. All statements and information are believed to be accurate and reliable, they are
3: Three phases are in mutual equilibrium; liquid + Na 2K + a solid solution rich in Na (peritectic line) • b 3 → b 4: The amount of solid compound increases until at b4 the liquid reaches its eutectic composition. It solidifies to give a two-phase solid of a solid solution rich in K and solid Na2K 5.9 Liquid-solid phase diagrams
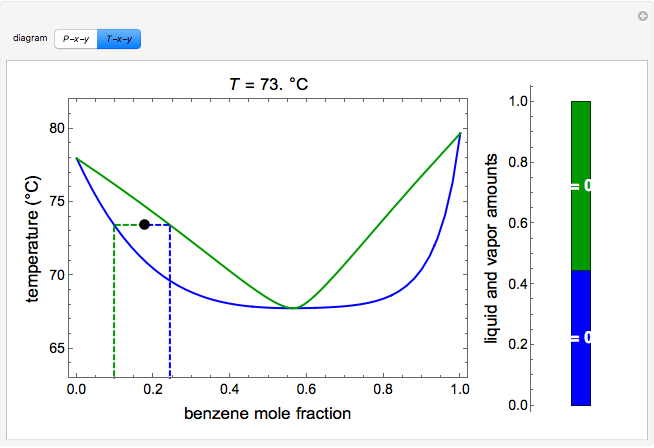
This Demonstration shows and diagrams for vapor-liquid equilibrium (VLE) of a benzene/ethanol mixture. This liquid mixture is non-ideal, and the system has an azeotrope (a condition where liquid and vapor have the same composition).
Vapor liquid equilibrium diagram.
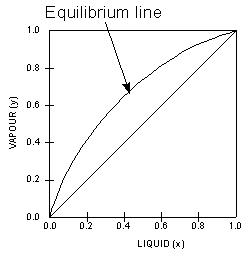
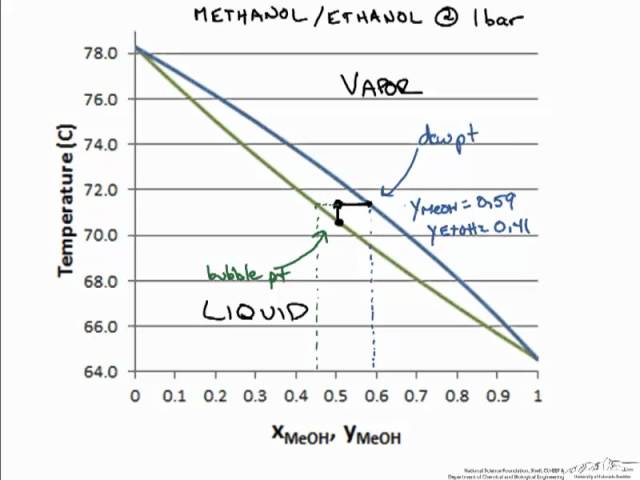
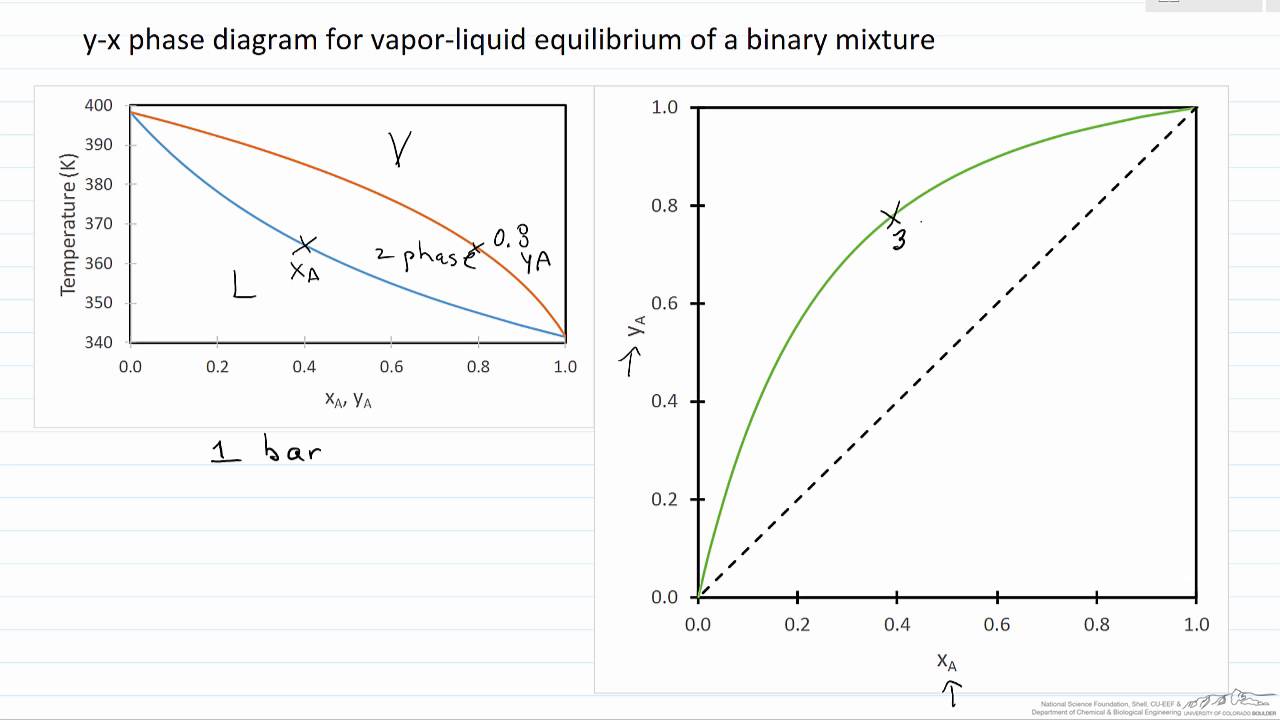
1.2 BINARY VLE PHASE DIAGRAMS Two types of vapor-liquid equilibrium diagrams are widely used to represent data for two-component (binary) systems. The first is a "temperature versus x and y" diagram (Txy). The x term represents the liquid composition, usually expressed in terms of mole fraction. The y term represents the vapor composition.
p-T diagram for a binary mixture. If a liquid phase is an ideal solution and a gaseous phase is a mixture of ideal gases, then the calculations may be executed ...
Vapor/Liquid Equilibrium • Previous chapters dealt with pure substances or with constant composition mixtures (air). • But in chemical reactions and number of industrially mass-transfer operations the . composition changes are the desired outcome. (Process such as distillation, absorption and extraction bring phases of different
Draw vapor-liquid equilibrium (VLE) and liquid-miscibility (LLE) phase diagrams, or calculate distillation processes. Over 300 organic compounds.
05/06/2014 · The vapor-liquid equilibrium (VLE) behavior of an -hexane/-octane mixture is demonstrated in and diagrams. The blue line represents the liquid-phase boundary (bubble point) and the green line represents the vapor-phase boundary (dew point).
Consider a binary mixture of ethanol and water. Vapor-liquid equilibrium (VLE) data can be computed using the modified Raoult's law: , where is the vapor pressure, is the total pressure, and are the liquid and vapor phase mole fractions of the light component (i.e., ethanol) when , and finally, is the activity coefficient. You can vary the pressure to any value between and (i.e., low …
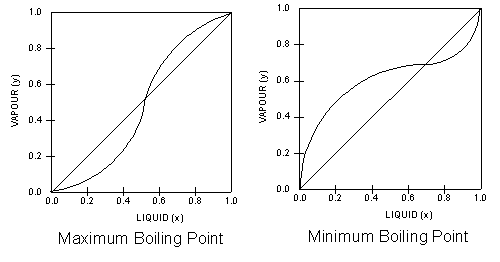
A liquid-vapor phase diagram plots temperature of mixture versus the mole fraction or composition. A phase diagram of 2 components that will have differing molecular interactions causing mixture's boiling point to rise/drop will show an azeotrope, in which there will be 2 lobes that either concave down/up depending on if there's positive ...
What is vapor liquid equilibrium diagram? Vapor-liquid equilibrium diagrams For each component in a binary mixture, one could make a vapor-liquid equilibrium diagram. Such a diagram would graph liquid mole fraction on a horizontal axis and vapor mole fraction on a vertical axis. What happens when a liquid vapor system at equilibrium?
A schematic three-dimensional diagram illustrating these surfaces for VLE is shown in Fig. 10.1. This figure shows schematically the P-T-composition surfaces which contain the equilibrium states of saturated vapor and saturated liquid for a binary system.
3.3 Phase Diagram for Water Vapor: Clausius-Clapeyron Equation. The Clausius-Clapeyron Equation. We can derive the equation for e s using two concepts you may have heard of and will learn about later: entropy and Gibbs free energy, which we will not go into here.Instead, we will quote the result, which is called the Clausius-Clapeyron Equation,
5.3, and the common tangent construction then gives the equilibrium vapor and liquid compositions. The phase diagram depends upon the Gibbs energies of ...
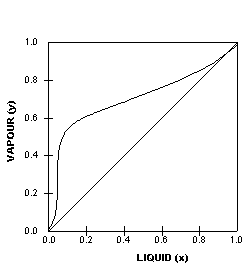
Summary: A liquid containing 12 mol% pentane (x = 0.12) could be heated to 370 K at which point it would start to boil (blue line).The saturated vapor (red line) in equilibrium with the boiling liquid is at the same temperature, but is richer in the more volatile component, n-pentane.The composition of this saturated vapor is 40 mol% n-pentane (y = 0.4).
Vapor-liquid Equilibrium Diagrams. Uses Simulink to compute Vapor-Liquid Equilibrium diagrams for ethanol-water binary mixture. To compute activity coefficients, program uses Van-Laar Model. This mixture presents a positive azeotrope. Requirements: · MATLAB Release: R12 · Simulink
Phase equilibrium is the study of the equilibrium which exists between or within different states of matter namely solid, liquid and gas. Equilibrium is defined as a stage when chemical potential of any component present in the system stays steady with time. Phase is a region where the intermolecular interaction is spatially uniform or in […]
In this video we will learn about fundamentals of vapor-liquid equilibrium and plotting the diagram in Aspen Plus.Email ID: cheme.friends@gmail.comInstagram:...
Vapor-liquid equilibrium data for Ethanol-Water* n-Buianol-Wator# Ethyl Aeeiate-Water# and n-Hexane-Ethanol at atmospheric pr 00 sura and £or n-Hoxana-Sthanol at 2f50 mnu, 59$ mm.* 1270 mm*# Ijbj mm** 2J10 mm.#
Vapor-Liquid Equilibrium Data. The experimental data shown in these pages are freely available and have been published already in the DDB Explorer Edition.The data represent a small sub list of all available data in the Dortmund Data Bank.For more data or any further information please search the DDB or contact DDBST.. Explorer Edition Data Main Page
In thermodynamics and chemical engineering, the vapor–liquid equilibrium (VLE) describes the distribution of a chemical species between the vapor phase and a liquid phase.. The concentration of a vapor in contact with its liquid, especially at equilibrium, is often expressed in terms of vapor pressure, which will be a partial pressure (a part of the total gas pressure) if any other gas(es ...
Drawing Vapor-Liquid Equilibrium Diagrams - posted in Student: I am asked to calculate and draw the vapor-liquid equilibrium (VLE) diagram of a binary mixture of acetone and glycerol, given only the total pressure, P = 1 atm (760 mmHg). It says for me to explicitly plot the mole fraction of acetone versus the mole fraction of glycerol. I have no idea where to start.
Apr 19, 2018 — in an n-hexane/n-octane mixture, then check solution.
Vapor-Liquid Equilibrium diagrams For each component in a binary mixture, one could make a vapor-liquid equilibrium diagram. Such a diagram would graph liquid mole fraction on a horizontal axis and vapor mole fraction on a vertical axis.
318 CHAPTER 10. Vapor/Liquid Eauilibrium: Introduction and KAC2, which represent the vapor pressure-vs.-T curves for pure species 1 and 2. More- over, the under and upper surfaces form a continuous rounded surface across the top of the diagram between Cl and C2, the critical points of pure species 1 and 2; the critical points of the
Figure 3 shows a vapor-liquid equilibrium diagram for the ethanol-water system at atmospheric pressure. The diagram shows mole percent ethanol in the liquid (X axis) vs mole percent ethanol in the vapor (Y axis). The plot could also be made for volume percent in the liquid vs volume percent in the vapor and the equilibrium Figure 1.
SIMPLE MODELS FOR VAPOR/LIQUID EQUILIBRIUM 3.1 Raoult's Law • Assumptions: - The vapor phase is an ideal gas (low to moderate pressure) - The liquid phase is an ideal solution (the system are chemically similar) *Chemically similar: the molecular species are not too different in size and are of the same chemical nature. eg: n-hexane/n ...
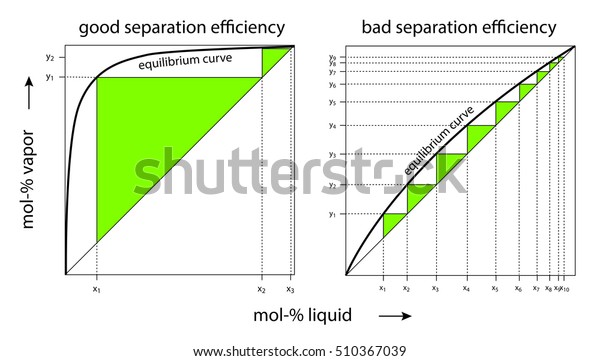
Vapour-Liquid-Equilibrium (VLE) Curves Constant pressure VLE data is obtained from boiling point diagrams. VLE data of binary mixtures is often presented as a plot, as shown in the figure on the right. The VLE plot expresses the bubble-point and the dew-point of a binary mixture at constant pressure.
equilibrium the partial molal free energies of the vapor and of the liquid are equal: F"(g) + RThf(g) = F"(2) + RTIna(2) (2) If the pressure is low enough so that the vapor acts as a Derfect eas (3) Here the pure-component term -AF"/RT is a function of temperature only, and the activity term is a function of
Uses an interactive simulation to explain how to read a T-x-y diagram for a binary mixture that obeys Raoult's law. This simulation is located at: https://w...
The vapor-liquid equilibrium (VLE) behavior of a benzene (C6H6)/toluene (C7H8) mixture is demonstrated in P-x-y and T-x-y diagrams. The blue line represents ...Sep 10, 2016
The basis of distillation is phase equilibrium—specifically, vapor-liquid equilibrium (VLE) and in some cases vapor-liquid-liquid equilibrium (VLLE). Distillation can only ... represents the vapor composition. The second diagram is a plot of x versus y. These types of diagrams are generated at a constant pressure. Because the pressure in a
Vapor Pressure Diagrams and Boiling Diagrams We are now ready to begin talking about phase diagrams involving two components. Our first few phase diagrams will involve only the liquid and gas (or vapor) phases. Later we will discuss two-component phase diagrams involving liquids and solids.
Calculation of vapor-liquid equilibrium (VLE) and drawing of phase diagrams. Name of substance. CAS-nr. Formula. Type of substance. acetone. 67-64-1. C 3 H 6 O. ketone.
A liquid mixture is partially vaporized and the vapor is allowed to come to equilibrium with the liquid. The process flow diagram is shown in Fig. 3.1. The vapor and liquid phases are then separated. Fig. 3.1 Flash distillation. Making a component i balance, FxiF = Vyi + Lxi = Vyi + (F - V)xi (3-3) Defining f = V/F, eq. (7.3) becomes
A liquid-vapor phase diagram of a binary system can be constructed by using a reflux apparatus. When a mixture of two soluble liquids is heated to a boiling point, the vapor phase is condensed and trapped in the pocket below the condenser.














0 Response to "35 vapor liquid equilibrium diagram"
Post a Comment