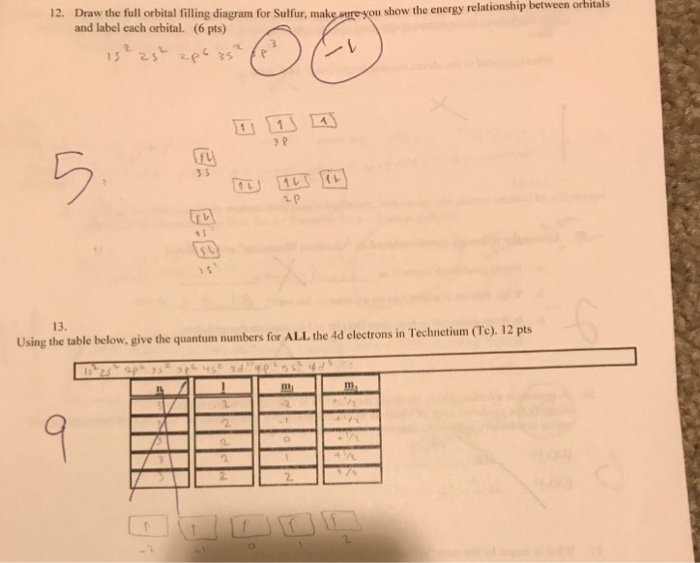
36 orbital filling diagram for sulfur
Orbital Filling Diagram for Sulfur. what is the orbital diagram for sulfur the orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each the arrows solved show the orbital filling diagram for s sulfur st answer to show the orbital filling diagram for s sulfur stack the sub shells in order of energy with the ... Orbital Diagram For Arsenic. Because the 4p section has 3 orbitals, but Arsenic ends with 4p3. It'll want to leave as few orbitals empty, so you have three arrows pointing up. The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org!
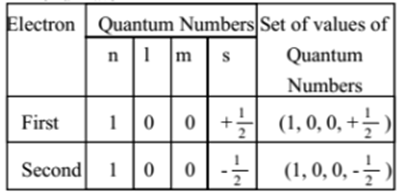
What is the electron configuration and orbital diagram for a phosphorus atom? What are the four quantum numbers for the last electron added? Solution The atomic number of phosphorus is 15. Thus, a phosphorus atom contains 15 electrons. The order of filling of the energy levels is 1s, 2s, 2p, 3s, 3p, 4s, . . .

Orbital filling diagram for sulfur
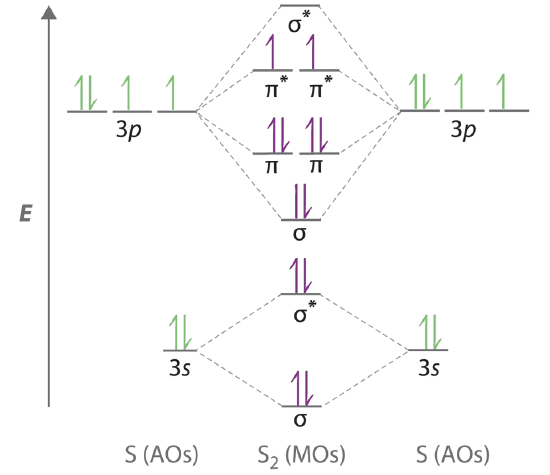
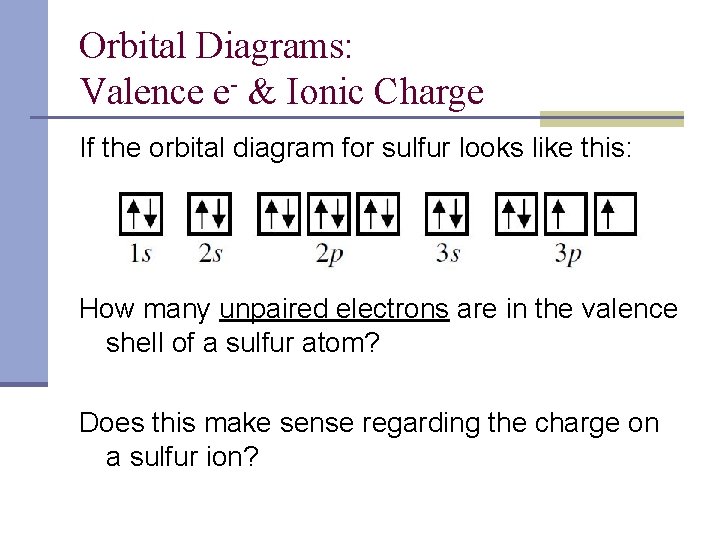
Nov 27, 2021 · H2S Molecular Orbital (MO) Diagram. The molecular orbital diagram of H2S can be explained in the following way. This is the MO diagram of H2S. The left-hand side will contain the atomic orbitals of sulfur i.e 3s2 3px2 3py1 3pz1. And on the right-hand side, there will be atomic orbitals of hydrogen. 8 valence electrons are filled in the MO orbitals. Write the orbital diagram for sulfur and determine its number of unpaired electrons. Electron configuration: 1s2 2s2 2p6 3s2 3p4. Orbital diagram: 1s= 1 up 1 down. 2s= 1 up 1 down. 2p= 1 up 1 down 1 up 1 down 1 up 1 down. 3s= 1 up 1 down. 3p= 1 up 1 down 1 up 1 up. Two unpaired electrons. Write the electron configuration for Ge. The orbital diagram in Model 3 is higher in energ than the ground state because ther is an electron in the 3 orbital that should be in a 2P orbital. The electron would need to have higher potential ennu to be in the 3s orbital. Read This! An state electron configuration is any electron configuration for an atom that contains the
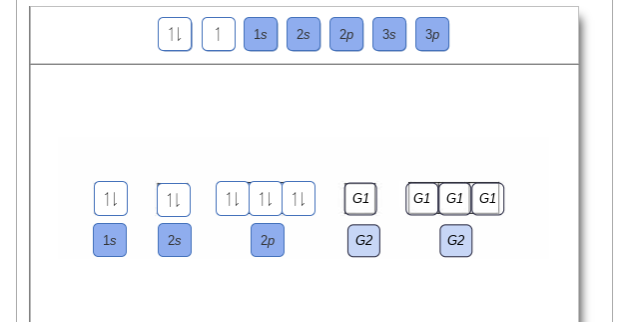
Orbital filling diagram for sulfur. 1 day ago · Groups one and two are the s-block, three through 12 represent the d-block, 13 to 18 are the p-block and the two rows at the bottom are the f-block. 41) Bromine has 28 core electrons. Show the orbital-filling diagram for S (sulfur). 5. Identify magnetic properties of atoms Ionization Energy Trends in the Periodic Table. Show the orbital-filling diagram for (bromine).Status: Resolved. Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top%(15). 1. Describe the two differences between a 2p x orbital and a 3p y orbital. An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box ... "s block" elements are filling the s orbital, p block elements have filled the s orbital and are adding electrons to the p orbitals. ... Sulfur : atomic number (Z) = … Question: Show the orbital-filling diagram for S (sulfur). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint(s) Reset Help 11 1 15 2s 2p 3s 3p G1 G1 G1 G1G1 G1 G1 G1 | G1 G2 G2 G2 G2 G2 Submit Part D Show the orbital-filling diagram for Br (bromine).
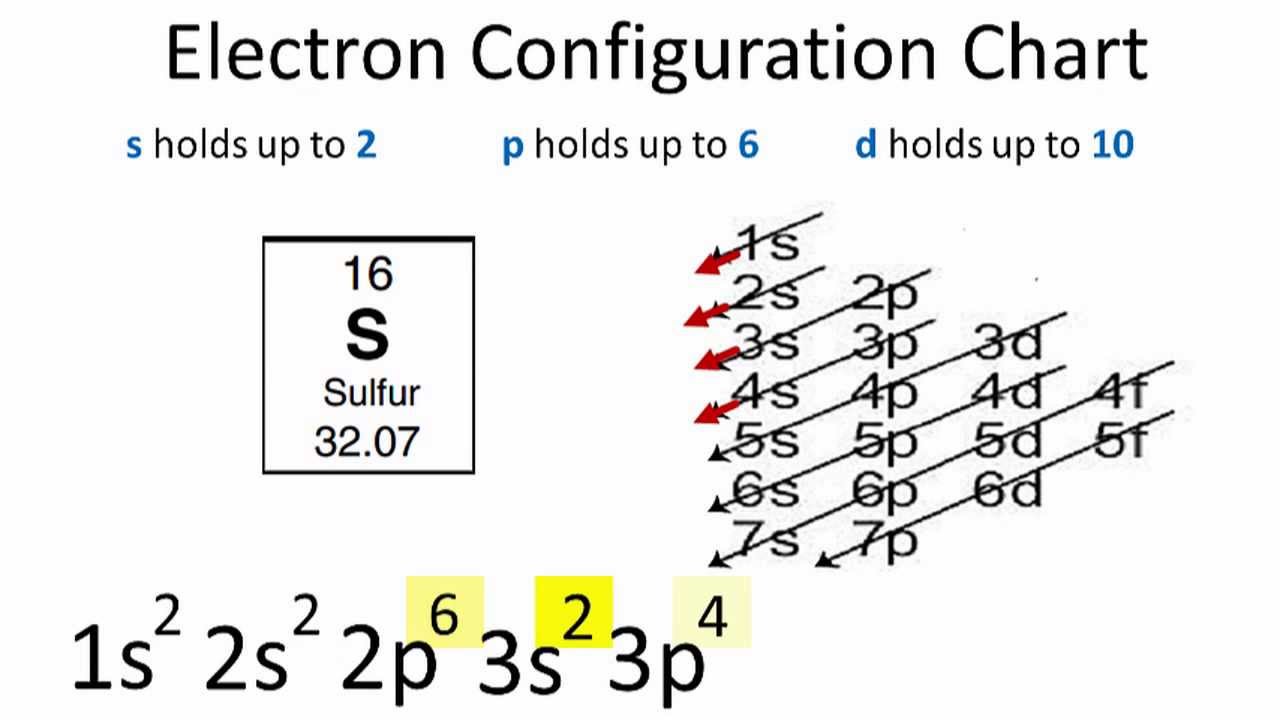
Mar 23 show the orbital filling diagram for sulfur. The bohr model is just 1 and the lewis diagram is just x h read more. The boxes represent sulfurs orbitals. In writing the electron configuration for sulfur the first two electrons will go in the 1s orbital. The arrows represent the 16 electrons of the sulfur atom and the directions represent ... Orbital Filling Diagram 02 Ex. Orbital filling diagram electron configuration electron dot diagram a. Electron configurations for the second period. ) Rule 2, 8, 8, 18 is a very Created Date: 5/13/2015 12:53:51 PM Chemistry Unit 2 Worksheet 1: Electron Configurations 1. Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium Where are the Electrons? Write the full electron configuration, short-hand electron configuration, and fill in the orbital diagrams, for the following elements. 1. Nov 01, 2021 · Orbital diagram of Sulfur (S) 17: Orbital diagram of Chlorine (Cl) 18: Orbital diagram of Argon (Ar) 19: Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: ... Electron configuration of elements Hund’s rule and Orbital filling diagrams. Categories Uncategorized Post navigation. Germanium (Ge) – Periodic Table (Element ...
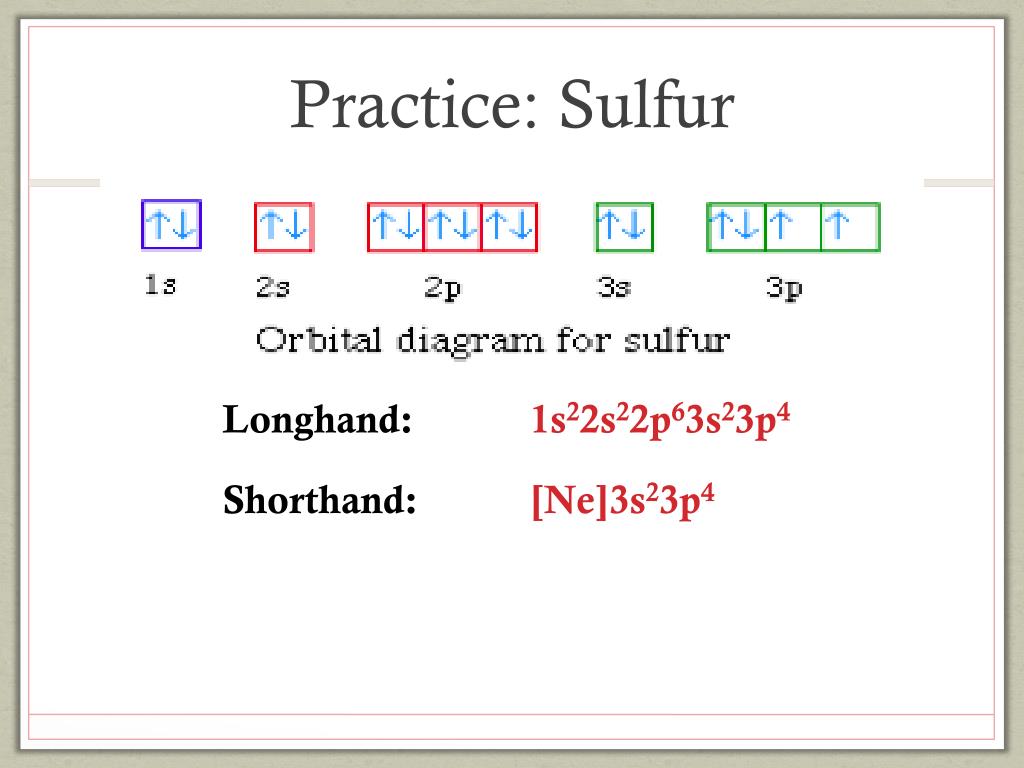
A Review Constants Periodic Table Learning Goal: To learn to create orbital-filling diagrams. An orbital-filling diagram shows the number of electrons in each orbital, which are shown in order of energy. The placement of electrons in orbitals follows a certain set of rules. Part C Show the orbital-filling diagram for S (sulfur). The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4. Nov 14, · Draw an orbital diagram for boron. Draw an orbital diagram for scandium Show the orbital-filling diagram for N (nitrogen). 1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following ... In the figure below, an atomic orbital diagram is used to illustrate the order of filling for the first ten electrons as shown by the numbers entered in the boxes. As an example, consider the electronic structure of sulfur. Since sulfur has 16 electrons, …
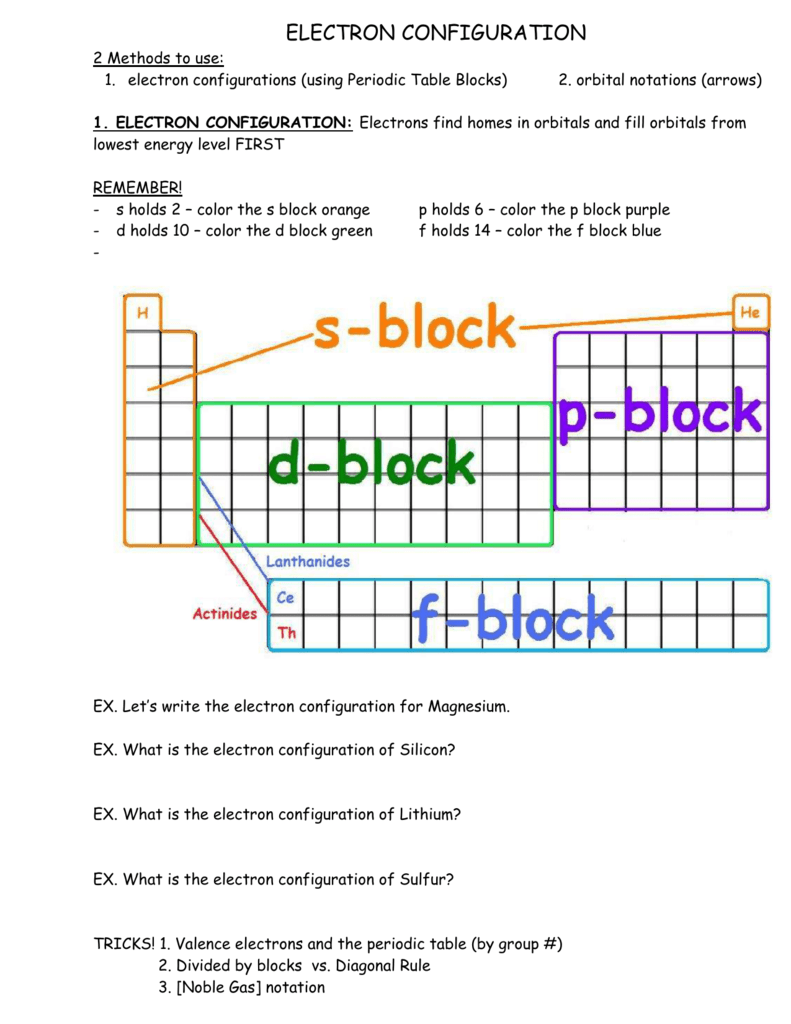
Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining four ...
The orbital diagram in Model 3 is higher in energ than the ground state because ther is an electron in the 3 orbital that should be in a 2P orbital. The electron would need to have higher potential ennu to be in the 3s orbital. Read This! An state electron configuration is any electron configuration for an atom that contains the
Write the orbital diagram for sulfur and determine its number of unpaired electrons. Electron configuration: 1s2 2s2 2p6 3s2 3p4. Orbital diagram: 1s= 1 up 1 down. 2s= 1 up 1 down. 2p= 1 up 1 down 1 up 1 down 1 up 1 down. 3s= 1 up 1 down. 3p= 1 up 1 down 1 up 1 up. Two unpaired electrons. Write the electron configuration for Ge.
Nov 27, 2021 · H2S Molecular Orbital (MO) Diagram. The molecular orbital diagram of H2S can be explained in the following way. This is the MO diagram of H2S. The left-hand side will contain the atomic orbitals of sulfur i.e 3s2 3px2 3py1 3pz1. And on the right-hand side, there will be atomic orbitals of hydrogen. 8 valence electrons are filled in the MO orbitals.
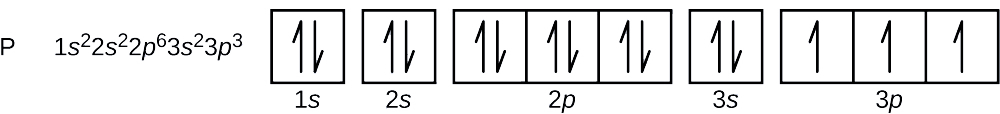









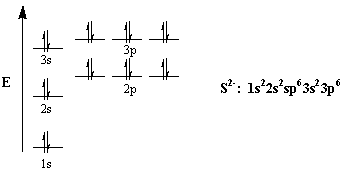







0 Response to "36 orbital filling diagram for sulfur"
Post a Comment