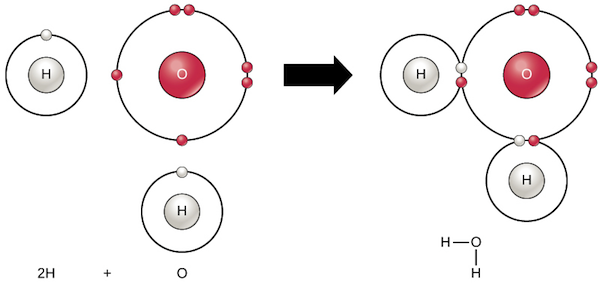
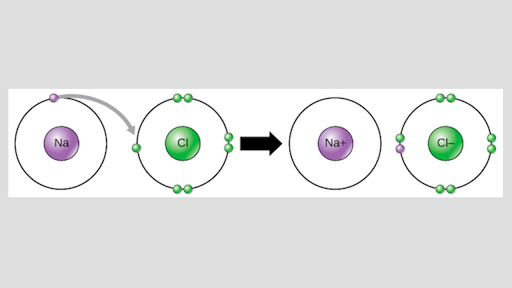
37 which type of chemical bond occurs when atoms share electrons, as shown in this diagram?
Ans. Valence electrons play an important role in chemical bonding. Atoms either lose, gain or share their valence electrons to form bonds. Q.4. What are the \(7\) valence electrons? Ans. When only seven electrons are present in the outermost shell of an atom, then the atom is said to possess seven valence electrons.
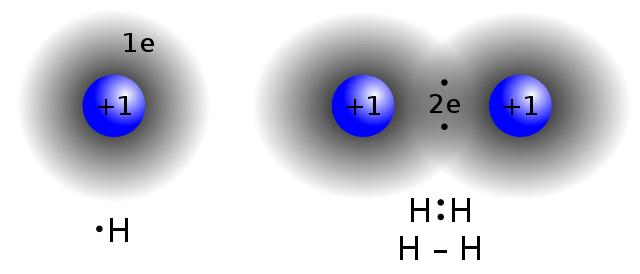
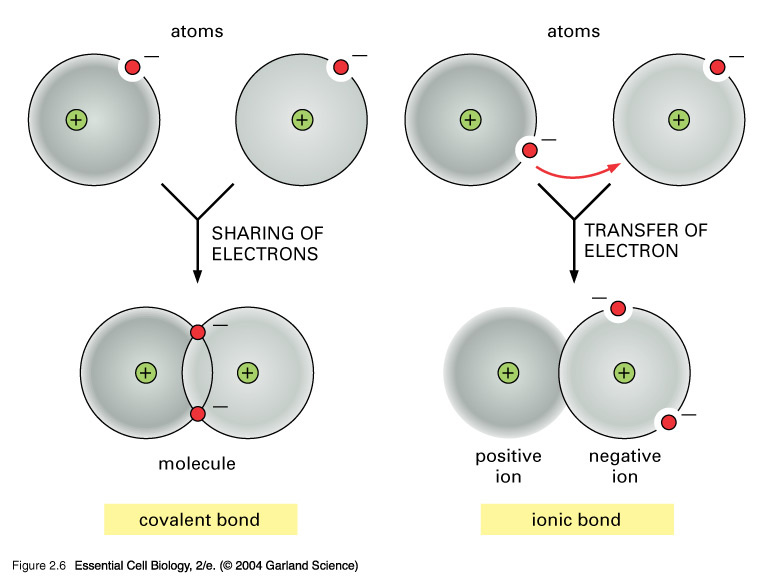
A covalent bond occurs when electron pairs are shared between two atoms; it is also the strongest of the chemical bonds. This sharing of the electrons helps the atoms reach their stable state. There are two main types of covalent bonds—non-polar and polar covalent bonds.
The important conditions for the Lewis structures are that every combining atom has to contribute at least one electron for sharing with other atoms and a chemical bond is formed owing to the sharing of electron pairs between atoms. The combining atom will achieve the nearest noble gas configuration by sharing electrons.
Which type of chemical bond occurs when atoms share electrons, as shown in this diagram?
The Lewis theory of chemical bonding helps us visualize the arrangement of atoms—how they are attached or bonded—in molecules. The valence electrons in each atom are the ones that participate in the bonding, and hence they are the only ones displayed in the Lewis structures.
Covalent bonds are formed when atoms share electrons. Lewis electron dot diagrams can be drawn to illustrate covalent bond formation. Double bonds or triple bonds between atoms may be necessary to properly illustrate the bonding in some molecules. Contributions & Attributions
CHAPTER 7. A (n) _______ bond is a chemical bond that results from sharing a pair of electrons between two atoms. A (n) ______ results from a transfer of one or more electrons from one atom or molecule to another. Nice work! You just studied 91 terms! Now up your study game with Learn mode.
Which type of chemical bond occurs when atoms share electrons, as shown in this diagram?.
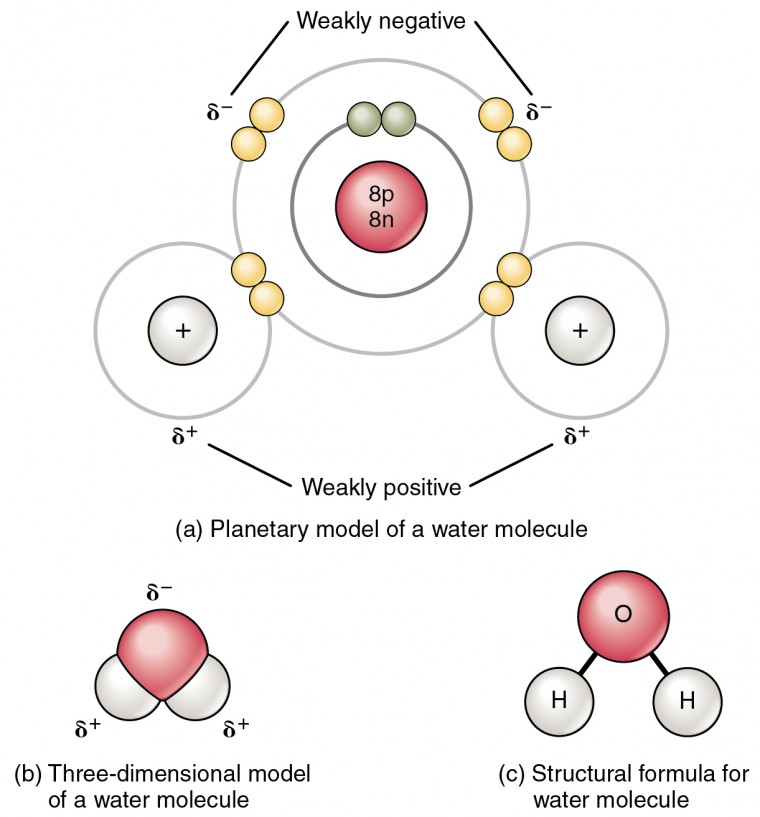
An ionic bond is a type of chemical bond in which the atoms have different electronegativity values from each other. For example, sodium (Na) and chlorine (Cl) form an ionic bond to make NaCl (table salt). However, in a covalent bond, the atoms are bound to share electrons. For example, if we talk about water ( H2O), it is a polar covalent bond.
It may share electrons with an adjacent atom to make a covalent bond, or it could take one electron away to form an ionic bond. As a result, halogens are the most reactive nonmetals, as they only require one electron to form bonds. To create a covalent link, they either remove an electron from another atom or share an electron from another storm.
The bond so formed between carbon and chlorine is a covalent bond since it is formed by sharing electrons. This makes CCl4 a covalent compound. As chlorine and carbon atoms share their outer electrons, chlorine attains the electronic configuration of argon and carbon attains that of neon.
Sep 4, 2021 — A chemical bond is a force of attraction between atoms or ions. Bonds form when atoms share or transfer valence electrons.
Covalent bonds result from the sharing of electrons between two atoms. The bonds typically involve one nonmetallic element with another Metallic bonds are found in solid metals (copper, iron, aluminum) with each metal atom bonded to several neighboring metal atoms and the bonding electrons are free to move throughout the 3-dimensional structure.
Nonpolar covalent bonds are a type of bond that occurs when two atoms share a pair of electrons with each other. These shared electrons glue two or more atoms together to form a molecule. Like...
At present, each atom has 7 electrons. Finally, after sharing three pairs of electrons that make the distribution of 6 electrons in a bond, it is known as a triple covalent bond. Hybridization of Nitrogen (N2) There are two types of bonds which are widely used in Chemistry, sigma (σ) and pi (π) bonds.
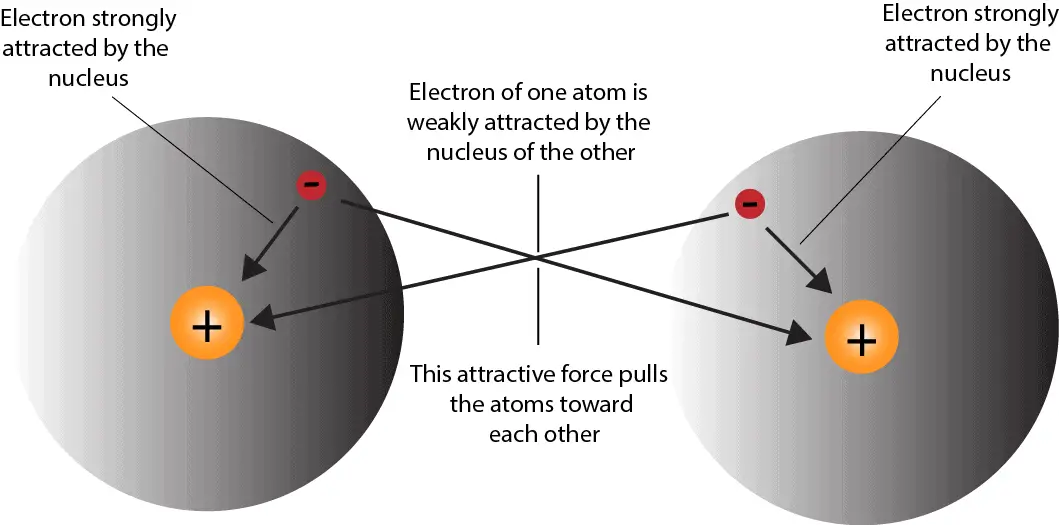
Covalent bonds are produced when electrons are shared between atoms and are attracted by both atoms' nuclei. In pure covalent bonds, the electrons are divided equally. In polar covalent bonds, the electrons are distributed unequally, as one atom employs a greater force of magnetism on the electrons than the other.
A chemical bond is the formation of chemical compounds by the sharing of electrons between atoms. Chemical bonding is the way through which molecules stay bound together. OR, if we elaborate it a little more, we should say, the force of attraction that holds the chemical constituents together to form a chemical compound is called chemical bonding.
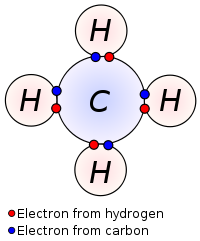
Each atom usually gives one electron in a bond pair. This bond pair is known as a covalent bond. For example A methane molecule is made by the mutual sharing of electrons between the carbon atom and hydrogen atoms. Coordinate (Dative) covalent bond
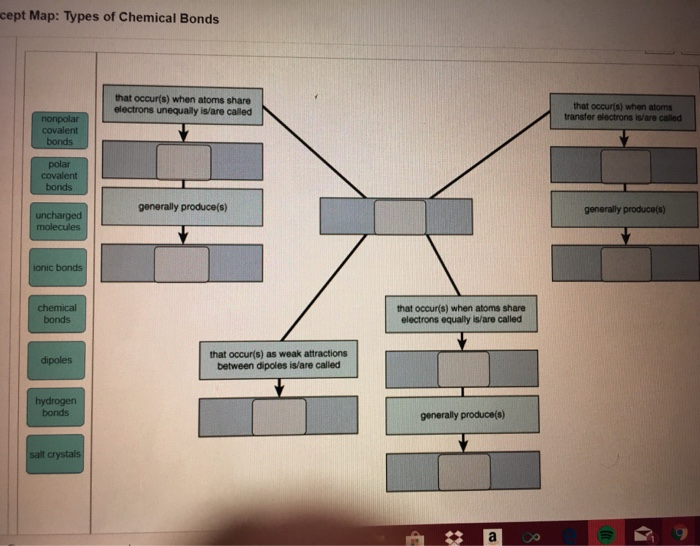
Chemical bonds include covalent, polar covalent, and ionic bonds. Atoms with relatively similar electronegativities share electrons between them and are ...
Covalent bonds are chemical bonds between two non-metal atoms A covalent bond between atoms is formed when are share buddy or more pairs of electrons. Lewis proposed that an atom forms enough covalent bonds to oak a weapon or closed outer electron shell during the diagram of methane shown here.
What is the intermolecular forces of HCl? HCl are made " polar covalent bonds " when atoms share electrons between hydrogen and chloride. Polar covalent bonds atoms such as hydrogen and chloride. HCl is made covalent bonds not hydrogen bonds.
There are two main types of chemical bonds in chemistry: covalent bonds and ionic bonds. Chemical bonds occur when two or more atoms either donate or share electrons. Electrons are negatively...
Which type of chemical bond occurs when atoms share electrons, as shown in this diagram? covalent. Which of the following is a mineral? salt (NaCl).
During bond formation, the orbitals of atoms are hybridized to share electrons with another atom. Here each Carbon atom forms three sigma bonds with Hydrogen atoms and one sigma bond with a Carbon atom. As a result, four orbitals that is 1s, px, py and pz orbitals are hybridized in each Carbon atom.
A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from the same atom. In the formation of a simple or ordinary covalent bond, each atom supplies at least one electron to the formation of the bond - but that is not the case every time.
Atoms can also make chemical bonds by sharing electrons equally between each ... A graph is shown with the x-axis labeled, “Internuclear distance ( p m ...
Polar bonds are the dividing line between pure covalent bonding and pure ionic bonding.Pure covalent bonds (nonpolar covalent bonds) share electron pairs equally between atoms. Technically, nonpolar bonding only occurs when the atoms are identical to each other (e.g., H 2 gas), but chemists consider any bond between atoms with a difference in electronegativity less than 0.4 to be a nonpolar ...
Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. The binding arises from the ...
The octet rule can be satisfied by the sharing of electrons between atoms to form covalent bonds. These bonds are stronger and much more common than are ionic bonds in the molecules of living organisms. Covalent bonds are commonly found in carbon-based organic molecules, such as DNA and proteins.
There are two different types of chemical bondings: metallic and covalent. In metallic bonding, atoms adopt the strategy of sharing electrons. This behavior is distinctive from ionic bonds wherein one atom takes an electron, and the other one gives it away.
Metallic bonding occurs between metal atoms. In this type of bond, the metal atoms each contribute their valence electrons to a big, shared, cloud of electrons.
The missing product of the reaction is BaCl2 and we can see that barium has two electrons on its outermost shell.. The complete reaction is Ba(ClO3)2 → BaCl2 + 3O2. From the reaction equation, we can see that the missing product is BaCl2. The compound is composed of one barium atom and two chlorine atoms.. Since chlorine has seven valence electrons, it means that it requires two electrons ...
A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is unequal. In a polar covalent bond, sometimes simply called a polar bond, the distribution of electrons around the molecule is no longer symmetrical.
Which refers to the type of chemical bond that involves the transfer of electrons between atoms? metallic polar covalent ... Sal drew the electron dot diagram of an aluminum atom as shown. ... Neon forms covalent bonds because it can share its valence electrons, but bromine has seven valence electrons and can gain only one more electron. ...
The two main types of chemical bonds are ionic bonds and covalent bonds, but there are some key differences between the two. Ionic vs. covalent bonding Whereas ionic bonds involve the complete transfer of electrons between atoms, covalent bonds are formed when two atoms share electrons.
Recall that covalent bonds are simply bonds formed between atoms in which electrons are unevenly shared. A coordinate covalent bond, sometimes referred to as a dative bond, is a special subtype of covalent bond that often forms between transition metals. In this type of covalent bond, both electrons in the bond are donated by a single atom.
Covalent bonds occur between identical atoms or between different atoms whose ... In this type of bond, each shared electron will be counted toward both ...
A valence electron is an electron that is attached to an atom and may be used to establish a chemical interaction; in a single covalent bond, both atoms contribute one valence electron to form a shared pair. The number of valence electrons can affect the chemical characteristics of an element as well as its ability to interact with other elements:






















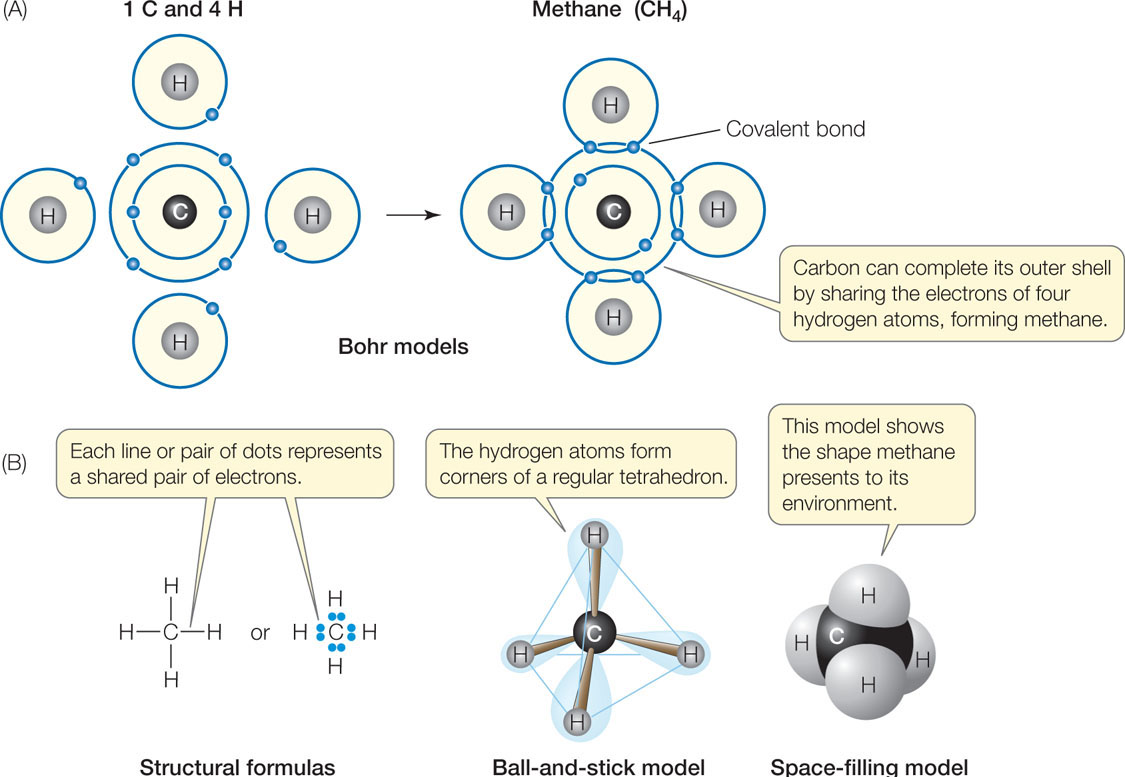

0 Response to "37 which type of chemical bond occurs when atoms share electrons, as shown in this diagram?"
Post a Comment