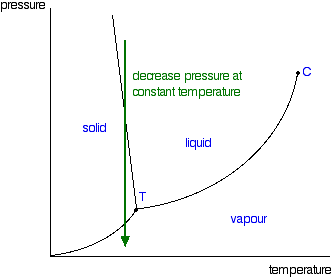
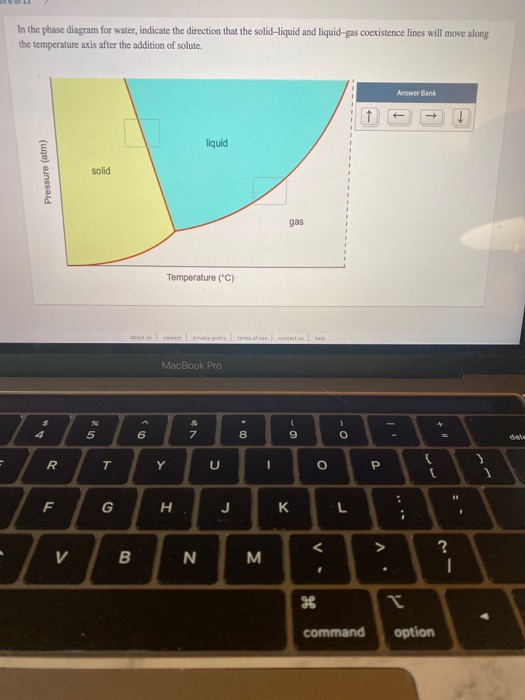
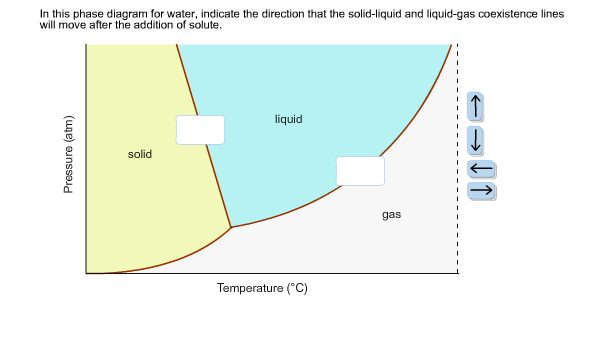
38 in this phase diagram for water indicate the direction that the solid-liquid and liquid-gas
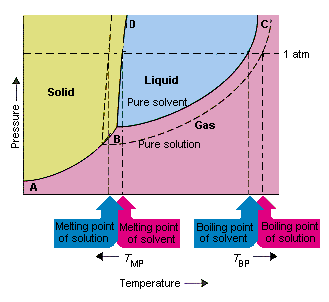
On a phase diagram this causes a crossing of both the solid-liquid boundary and the liquid-gas boundary. Which of the following best describes what happens? Refer to the phase diagram for carbon dioxide in Problem Set 60: Phase Diagrams, in the Chemistry: Problems and Solutions book. In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. 15. Menthol is a crystalline substance with a peppermint taste and odor.
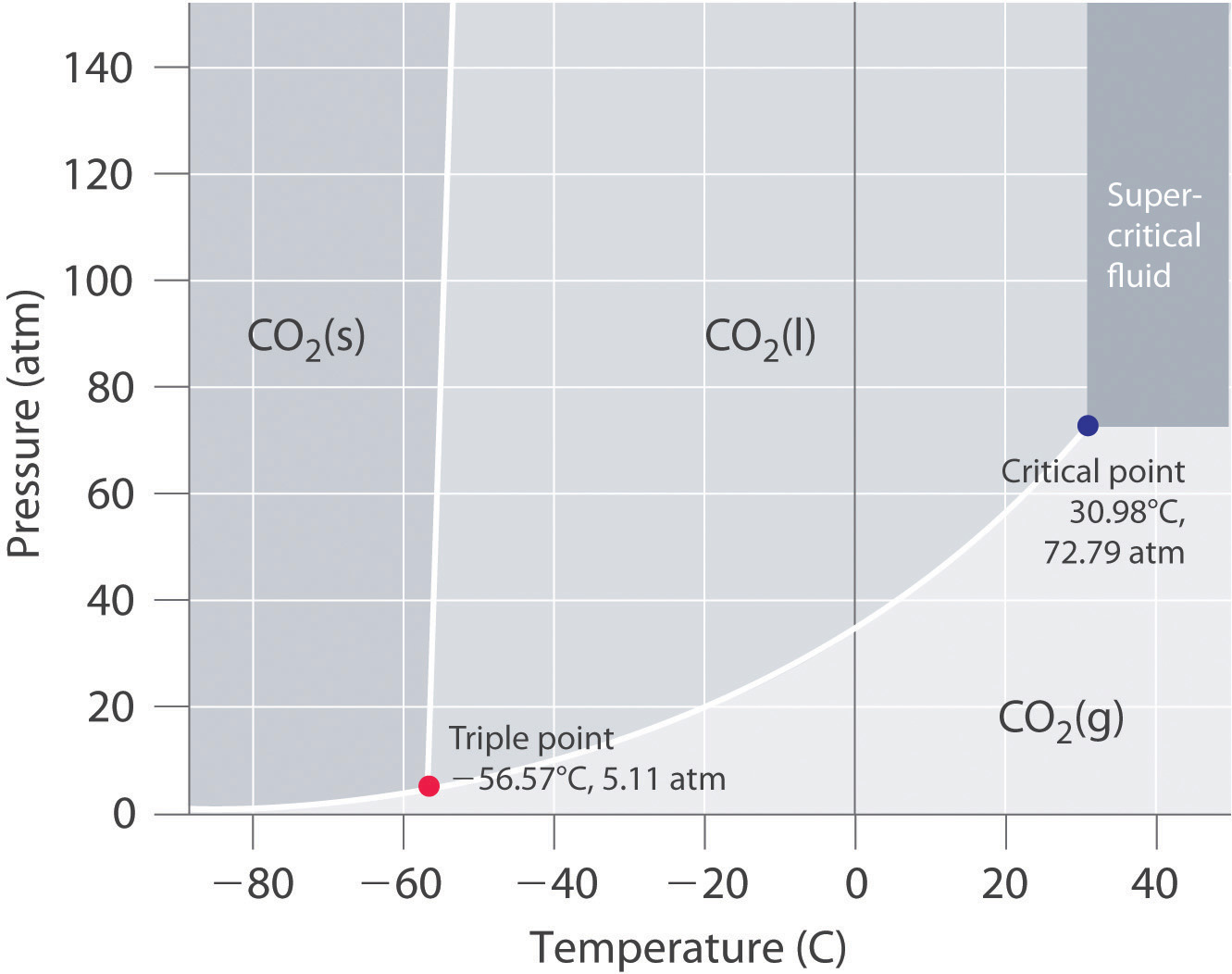
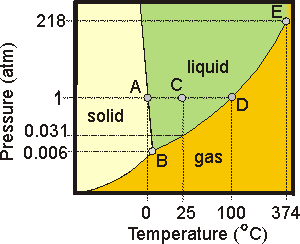
Express your answer as two letters ordered in the direction of melting. Be careful to put the letters in the correct order. Answer: AH. Part G: One of the most important points on a phase diagram is the triple point, where gas, liquid, and solid phases all can exist at once.

In this phase diagram for water indicate the direction that the solid-liquid and liquid-gas
Chapter 8 Phase Diagrams. (b) The interpretation of diagrams. Point a represents the vapor pressure of a mixture with liquid composition xA and b represents the composition of the vapor that is in equilibrium with the liquid at that pressure. Note that when two phases are in equilibrium, P = 2, so F' = 1. In the phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move along the temperature axis after the addition of solute. Answer Bank liquid solid gas Temperature (°C) Pressure (atm) check_circle. A distinct boundary between the more dense liquid and the less dense gas is clearly observed. As we increase the temperature, the pressure of the water vapor increases, as described by the liquid-gas curve in the phase diagram for water (), and a two-phase equilibrium of liquid and gaseous phases remains. At a temperature of 374 °C, the vapor ...
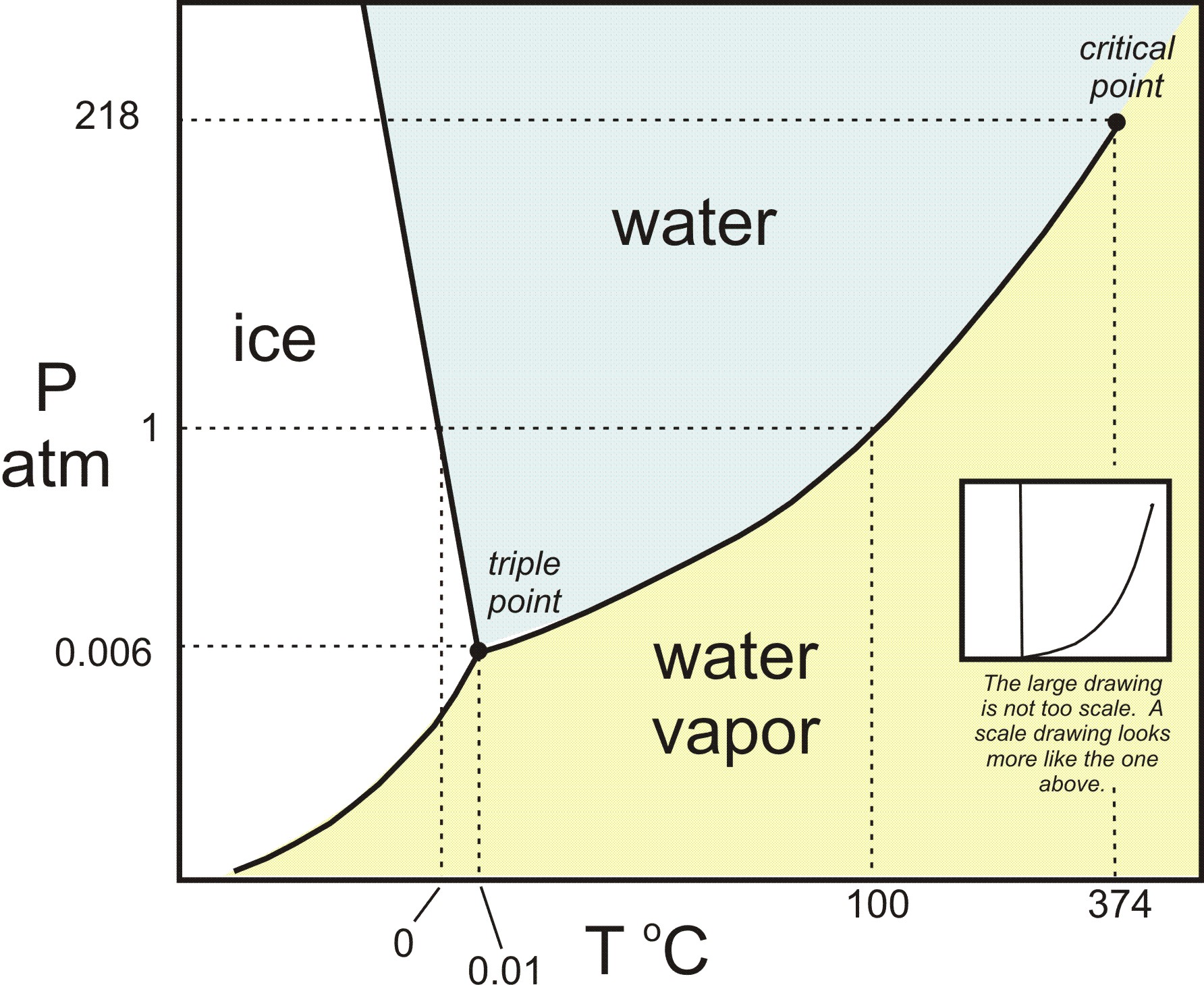
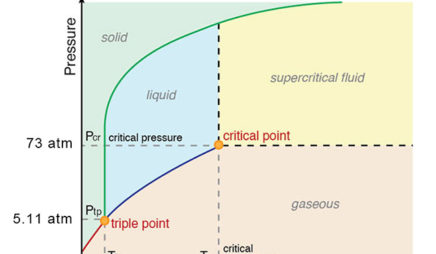
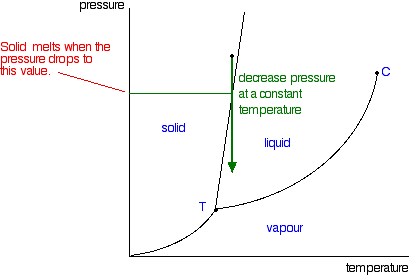
In this phase diagram for water indicate the direction that the solid-liquid and liquid-gas. the conditions under which the solid and liquid phases may coexist. These conditions are graphically presented in equilibrium phase diagrams, which can be experimentally determined. 2. THE ONE-COMPONENT PHASE DIAGRAM . Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. Phase diagram of water Note: for H2O melting point decreases with increasing pressure, for CO2 melting point increases with increasing pressure. ... Melting point - temperature at which a substance turns from solid to liquid. solid liquid gas. The three phase changes can be brought about by changes in temperature or pressure: Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ... Unlike most materials, water is more dense as a liquid than as a solid. So increasing pressure favors the liquid instead of the solid. Because water tends to form hydrogen bonds, its (ordinary) solid phase does not have a close-packed structure. The molecules in water ice take up extra space in order to make their hydrogen bonds line up and make the most stable solid at ambient pressure: (from ...
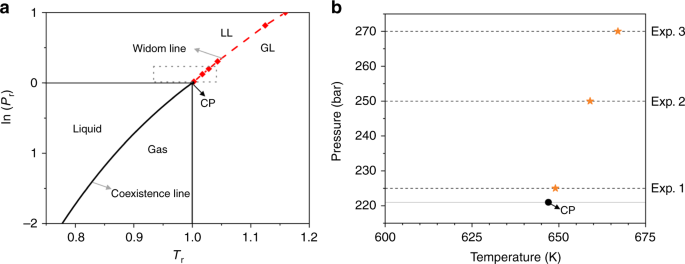
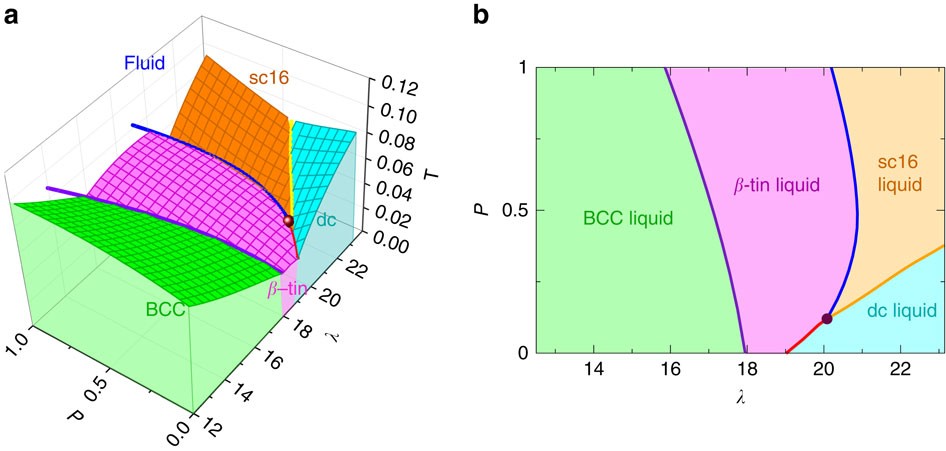
A phase diagram for an organic compound could include mesophases, which are intermediate phases between a solid and a liquid. Mesophases are of particular interest for liquid crystal technology. While phase diagrams look simple at first glance, they contain a wealth of information concerning the material for those who learn to read them. This unusual feature of water is related to ice having a lower density than liquid water. Increasing the pressure drives the water into the higher density phase, which causes melting. Another interesting though not unusual feature of the phase diagram is the point where the solid-liquid phase line meets the liquid-gas phase line. In the phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move along the temperature axis after the addition of solute. Answer Bank 1. → liquid Pressure (atm) solid gas Temperature (°C) Question: In the phase diagram for water, indicate the direction that the solid-liquid and liquid ... S gas T=T 1 = const gas+liquid liquid liquid+solid solid Because molecules aggregate differently in different phases, we have to provide (or remove) energy when crossing the coexistence curves. The energy difference is called the latent heat; crossing the coexistence curve, the system releases (absorbs) a latent heatL.
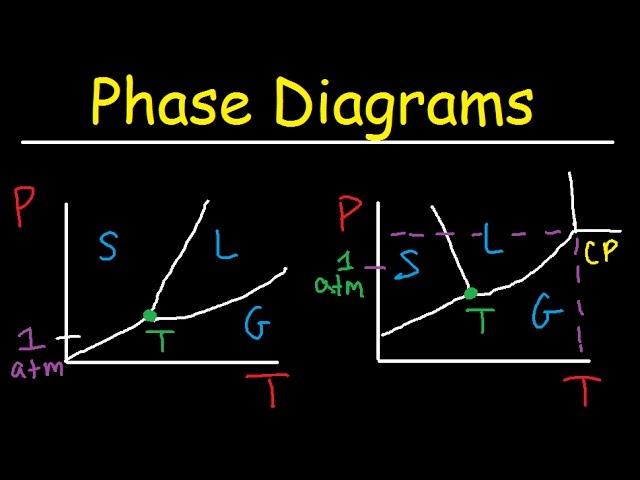
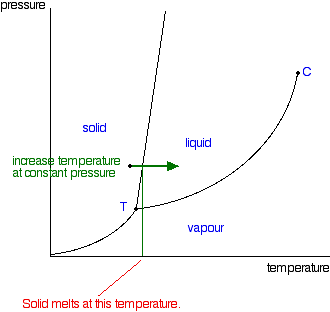
Phase diagrams. A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance. Solid Liquid Phase Diagrams Salt Solution For each of the following cases name the solution into which a net flow of water if any will occur. In this phase diagram for water indicate the direction that the solid liquid and liquid gas. At 2 it would be a liquid. When moving between solid to gas phases the material undergoes sublimation. What would be the relative lengths on the qp axis Of the liquid + solid, liquid, and liquid + gas segments for water if the drawing were to scale and the system consisted ofH20? For the liquid + solid segment, the length of the segment is AH for the liquid segment, the fusion length is and for the liquid + gas segment, the length is AHvaporiza1ion. When a solid is heated at constant pressure, it melts to form a liquid, which eventually boils to form a gas. Phase diagrams can be used in several ways. We can focus on the regions separated by the lines in these diagrams, and get some idea of the conditions of temperature and pressure that are most likely to produce a gas, a liquid, or a solid.
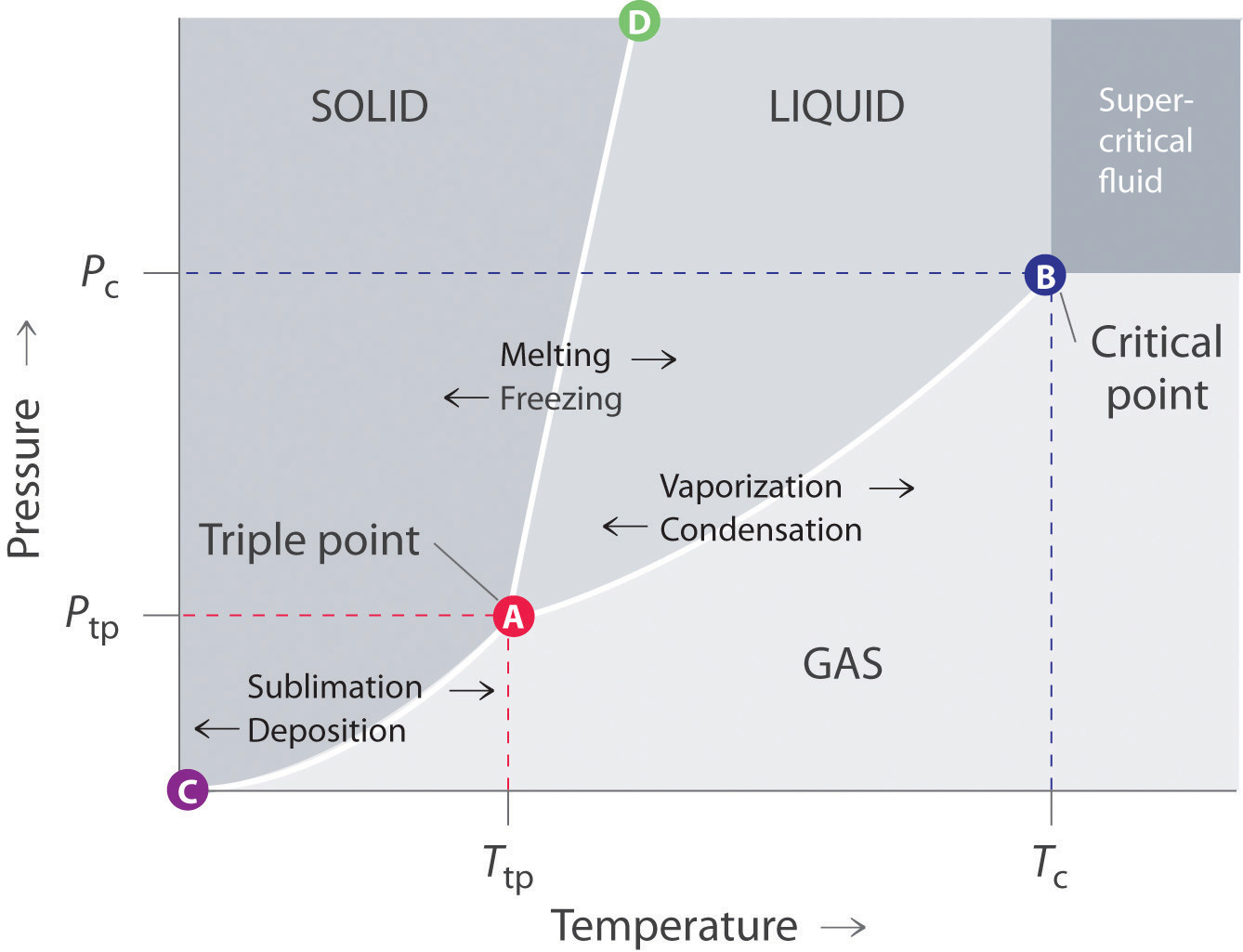
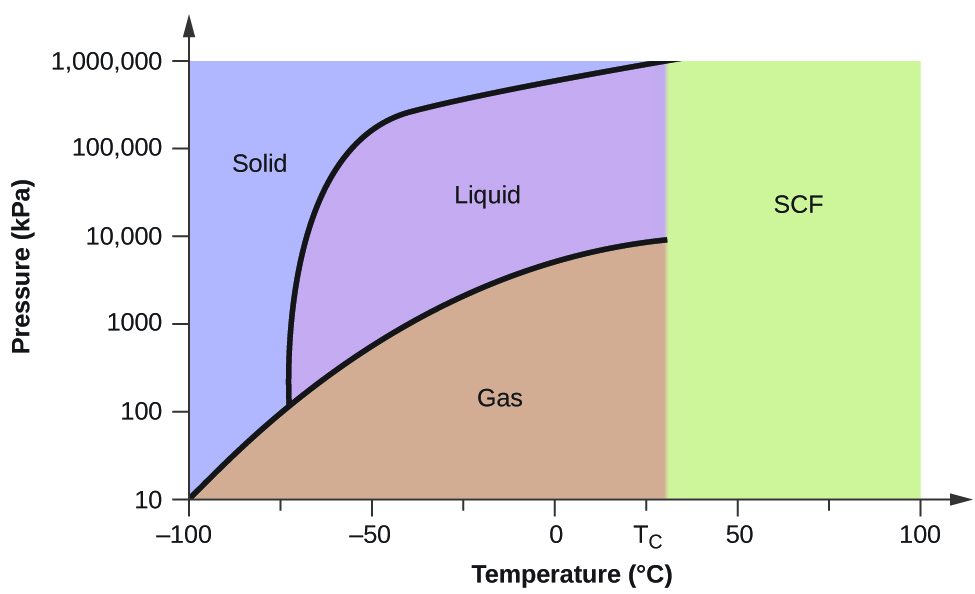
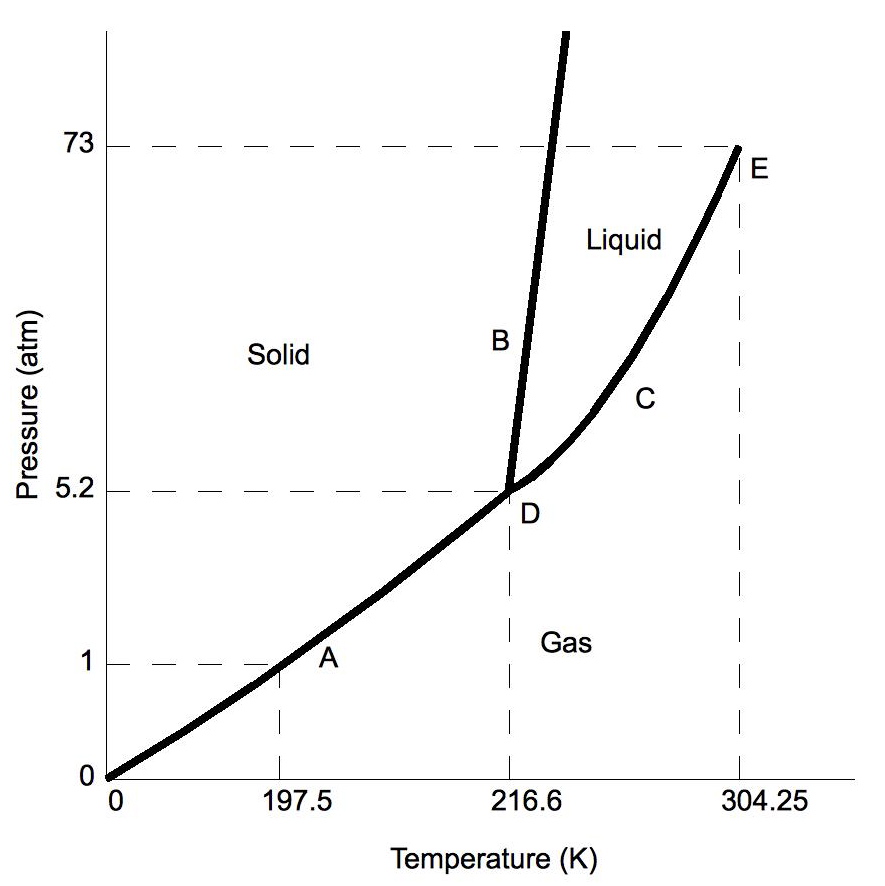
The solid liquid line is "normal" (meaning positive sloping). For this, complete the following: 1. Roughly sketch the phase diagram, using units of atmosphere and Kelvin. Answer. 1-solid, 2-liquid, 3-gas, 4-supercritical fluid, point O-triple point, C-critical point -78.5 °C (The phase of dry ice changes from solid to gas at -78.5 °C) 2.
This is the phase diagram for water. So just to understand what's going on here, is that on this axis, I have pressure. On the x-axis, I have temperature, and at any given point, this diagram will tell you whether you're dealing with a solid, so solid will be here, a liquid will be here, or a gas.
Problem. : In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. FREE Expert Solution. After the addition of solute, the solid - liquid coexistence line will move towards lower temperatures. 95% (314 ratings)
12.4: Phase Diagrams. To understand the basics of a one-component phase diagram as a function of temperature and pressure in a closed system. To be able to identify the triple point, the critical point, and four regions: solid, liquid, gas, and a supercritical fluid. The state exhibited by a given sample of matter depends on the identity ...
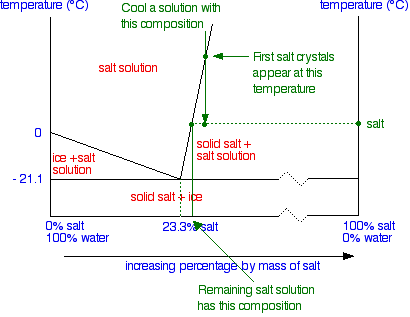
(liquid) + S (solid sugar) water-sugar system. Chapter 9 - 5 Phase Equilibrium Equilibrium: minimum energy state for a given T, P, and composition (i.e. equilibrium state will persist indefinitely for a fixed T, P and ... Phase Diagrams • Indicate phases as function of T, Co, and P.
In the phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move along the temperature axis after the addition of solute. Calculate the osmotic pressure of a 0.186 M aqueous solution of sucrose, C12H22O11, at 37*C.
In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. Question: In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute.
Similarly, if we heat a volume of water above 100 degrees Celsius, or 212 degrees Fahrenheit, water changes its phase into a gas called water vapor. Changes in the phase of matter are physical changes, not chemical changes. A molecule of water vapor has the same chemical composition, H2O, as a molecule of liquid water or a molecule of ice.
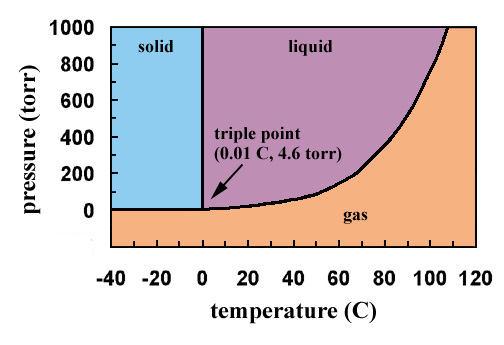
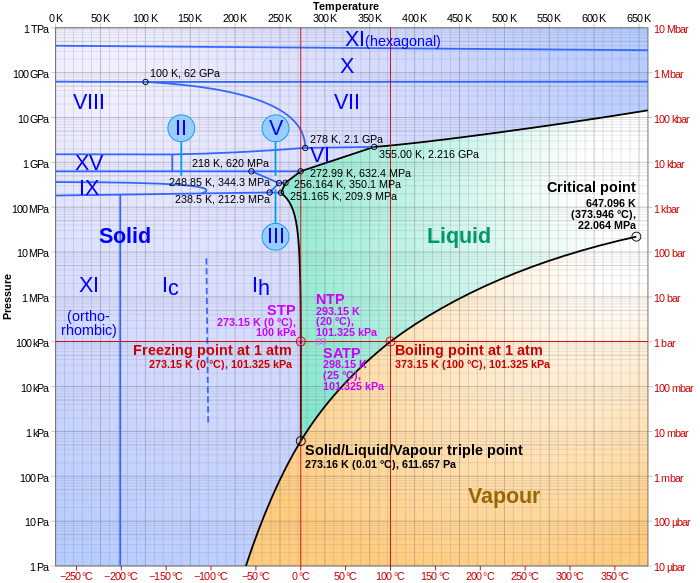
The phase diagram for water is shown below. The solid lines identify the temperatures and pressures at which an equilibrium exist between phases. The point at which the lines intersect represents the triple point. At the pressure and temperature of the triple point, all three phases (solid, liquid and gas) exist in equilibrium. The triple point ...
Vaporization gas liquid. In this phase diagram for water indicate the direction that the solid liquid and liquid gas. Melting liquid solid. Menthol is a crystalline substance with a peppermint taste and odor. Triple point the temperature at which a solid liquid and gas phases coexist in equilibrium. Deposition liquid gas.
The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water.The axes correspond to the pressure and temperature.The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.. The curves on the phase diagram show the points where the free energy (and other ...
A distinct boundary between the more dense liquid and the less dense gas is clearly observed. As we increase the temperature, the pressure of the water vapor increases, as described by the liquid-gas curve in the phase diagram for water (), and a two-phase equilibrium of liquid and gaseous phases remains. At a temperature of 374 °C, the vapor ...
In the phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move along the temperature axis after the addition of solute. Answer Bank liquid solid gas Temperature (°C) Pressure (atm) check_circle.
Chapter 8 Phase Diagrams. (b) The interpretation of diagrams. Point a represents the vapor pressure of a mixture with liquid composition xA and b represents the composition of the vapor that is in equilibrium with the liquid at that pressure. Note that when two phases are in equilibrium, P = 2, so F' = 1.






:max_bytes(150000):strip_icc()/phase_diagram_generic-56a12a1b5f9b58b7d0bca817.png)






0 Response to "38 in this phase diagram for water indicate the direction that the solid-liquid and liquid-gas"
Post a Comment