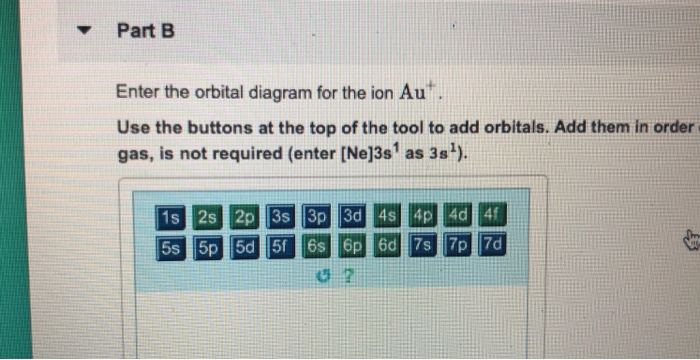
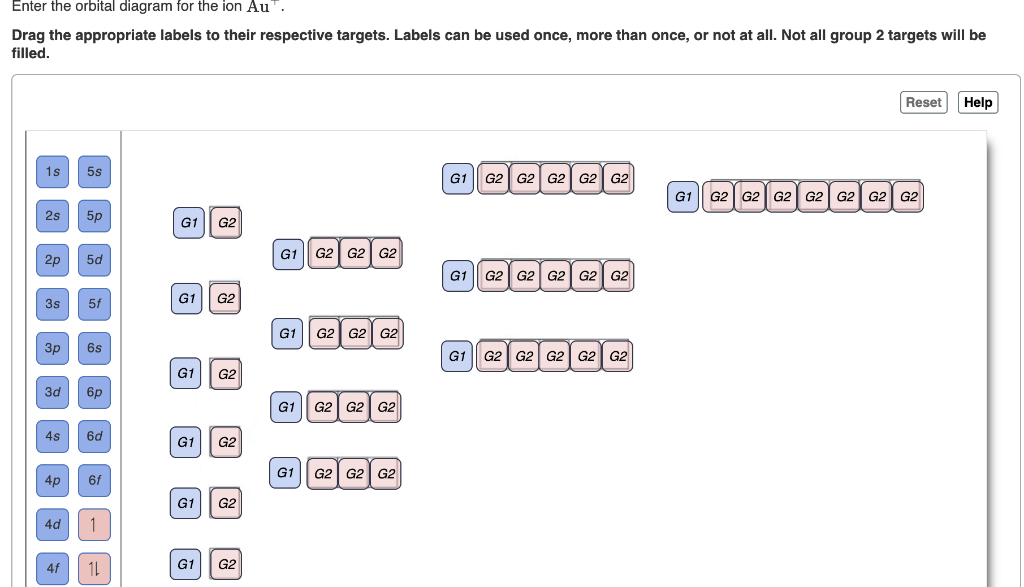
38 write orbital diagram for au+.
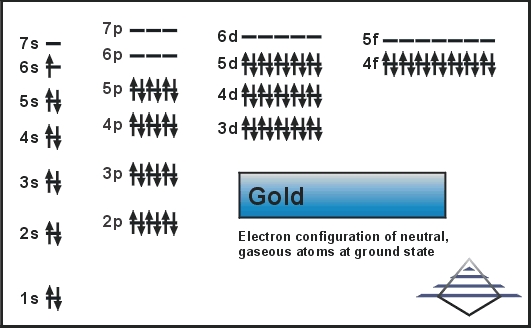
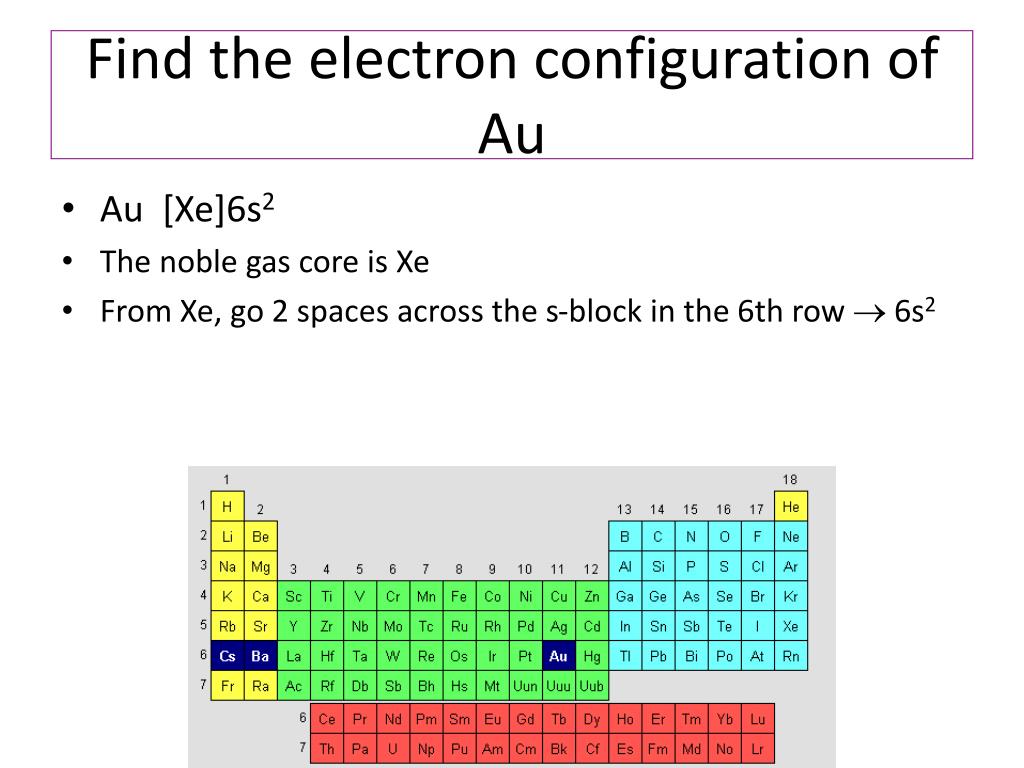
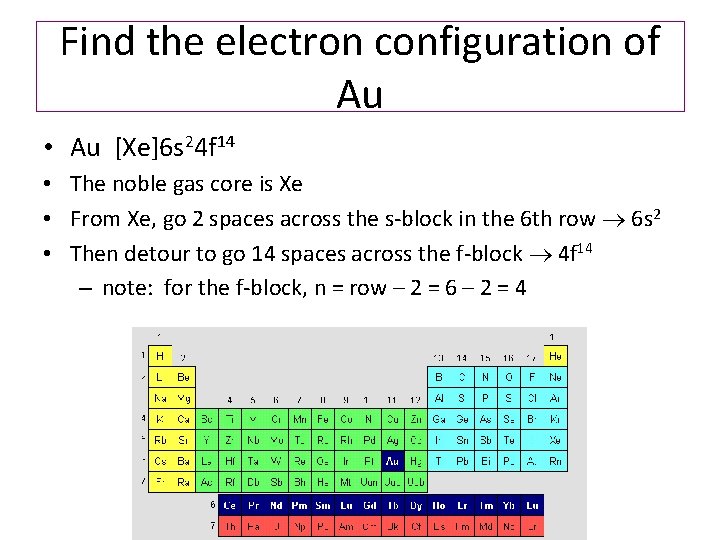
Molecular Orbital: Graphical representation of a molecule is done by following three rules, the Aufbau Principle, Hund's rule and the Pauli-Exclusion principle ...1 answer · Top answer: The give cation is Au+Au+ named as gold ion. The atomic number in periodic table is 79. Electronic configuration of gold is: [Xe]4f5d106s1[Xe]4f5d106s1 ...
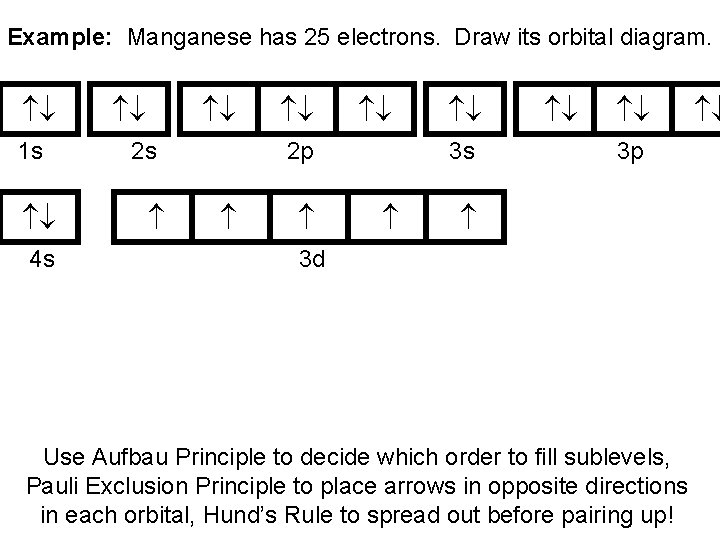
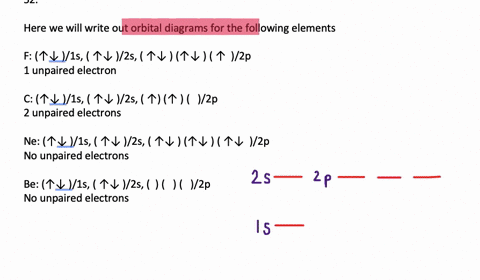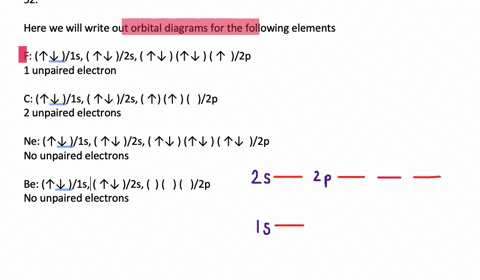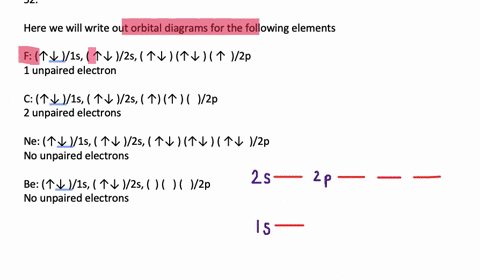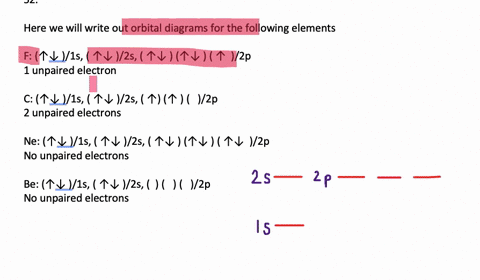
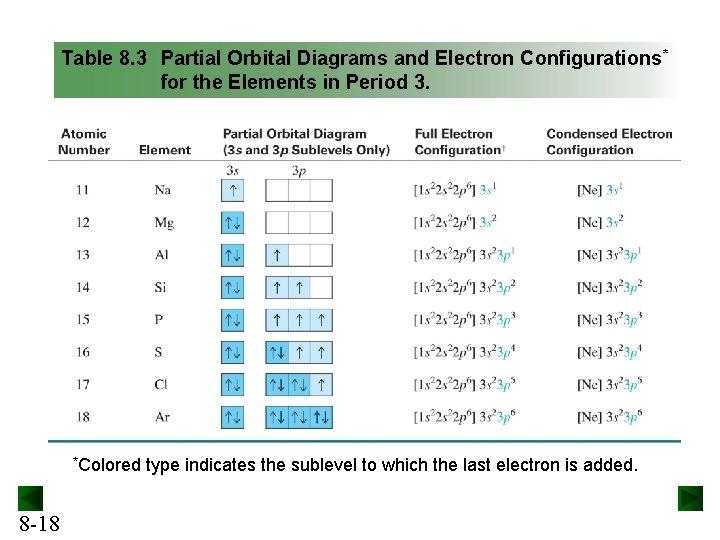
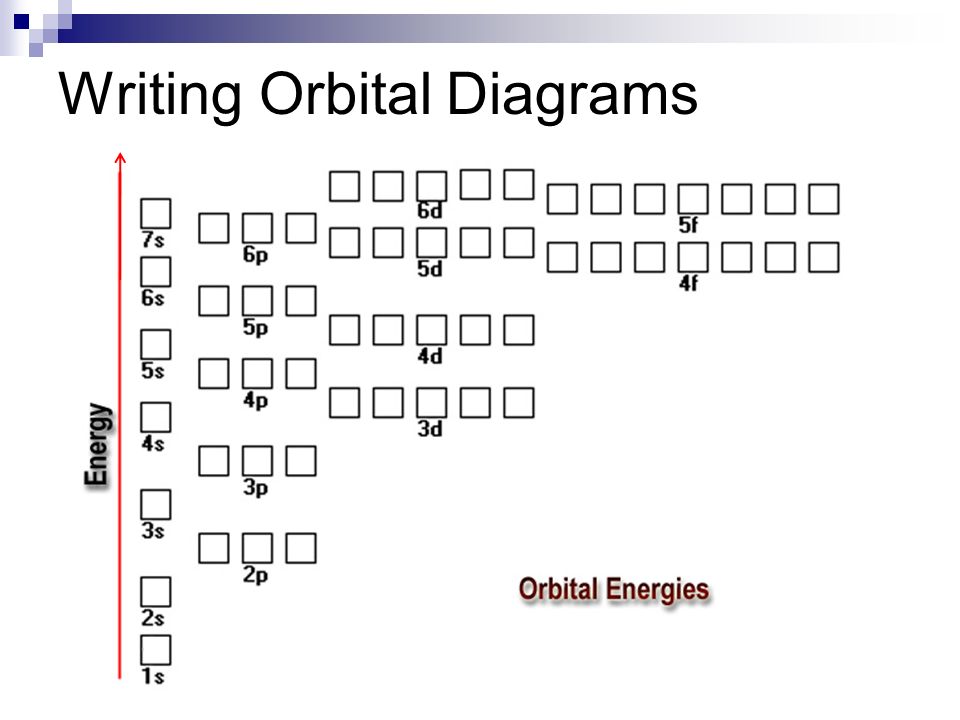
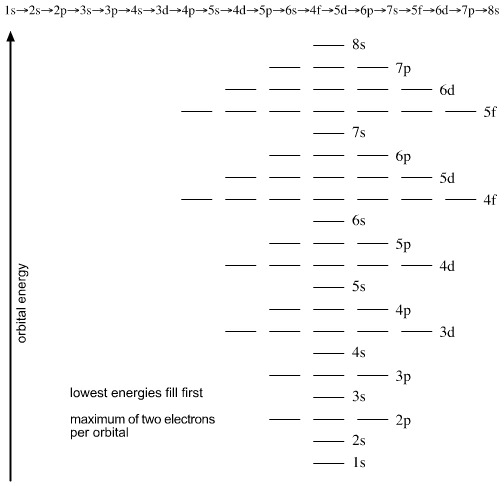
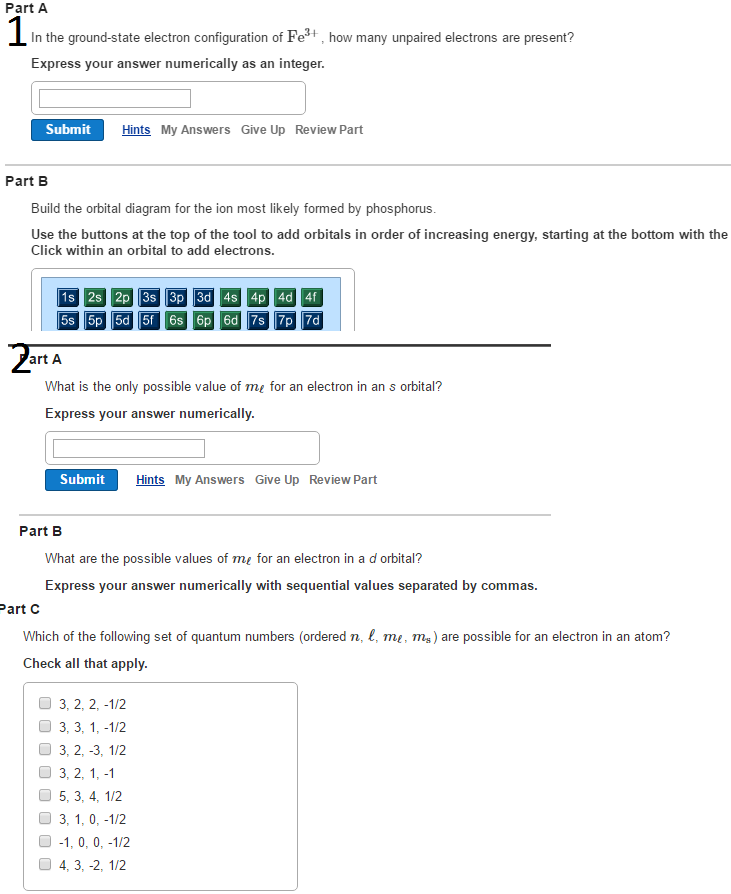
In addition to listing the principle quantum number, n, and the subshell, ℓ, the orbital diagram shows all the different orientations and the spin of every electron. The diagram shows the number of subshell by using boxes or lines for electrons (use three for p-orbitals, five for d-orbitals, and 7 for f-orbitals).
Gold (Au) ; Electrons Per Shell, 2 8 18 32 18 1 ; Electron Configuration, [Xe] 4f14 5d10 6s ; Orbital Diagram. 1s. ↿⇂. 2s. ↿⇂. 2p. ↿⇂. ↿⇂. ↿⇂. 3s. ↿⇂.
Write orbital diagram for au+.
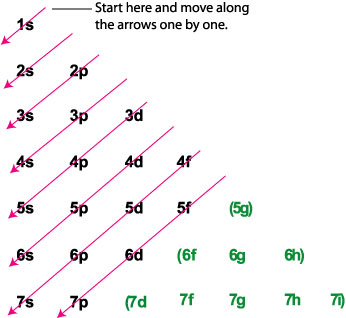
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. Arrows (or half arrows) are used to represent the electrons occupying the orbitals. ... They are: However, one can write the electronic configuration just by understanding the Aufbau principle. It states that, in ground state, the ...
Question: Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. This problem has been solved! See the answer ...
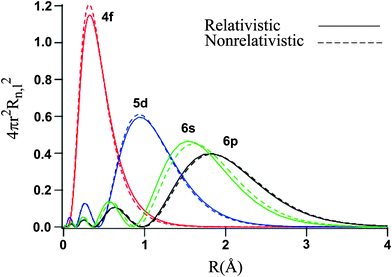
Relativity says if faster than light, then backwards in time. Diagram 3 one electron traveling between two points undergoes a delay, but Diagram 4 when going faster than light it goes backwards in time again. Diagram 5, one electron on its travel may create an electron-positron pair from empty space, and a virtual positron travels forward in time.
Write orbital diagram for au+..
🔴 Answer: 2 🔴 on a question The answer is 9x^2 - 12y^2 - 11x, i got it off of Quizlet. - the answers to answer-helper.com
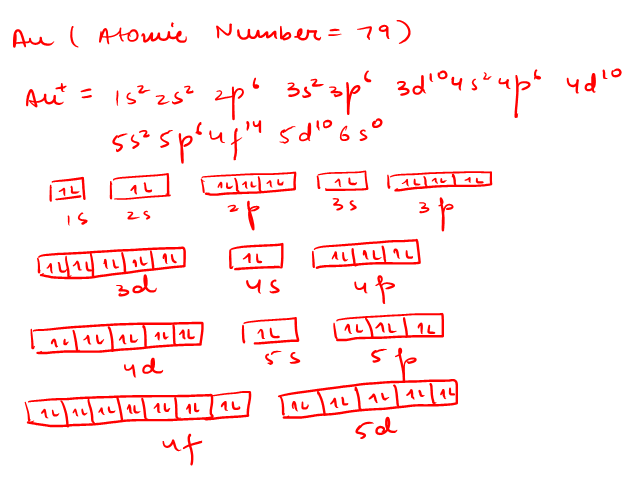
In each box can be two arrows with opposite spin maximum! ... Since Au+ has lost one electron and the numbers (or arrows in the diagram) represent the electrons, ...2 answers · Top answer: orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 you just ...
Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. Feb 08 2021 08:54 PM.
enter image description here In the above diagram - is a set of bulbs (destination), power source & wires. Color coding is used to understand the current flow. We have to write the following method which should return true or false whether the source is connected to the destination or not Bulb - We can have multiple bulbs Source - We can have a single source Wire - in a block; wire can ...
Jun 24, 2021 — Since Au+ has lost one electron and the numbers (or arrows in the diagram) represent the electrons, its configuration is: 1s2 2s2 2p6 3s2 3p6 ...
Nov 28, 2020 — Get the detailed answer: Write the orbital diagram for Au+. Determine if the ion is paramagnetic or diamagnetic.1 answer · Top answer: Gold (Au) is element 79 and is located in Period 6, the second to the last element of the 5d transition metals. It It is the ninth element in thes series ...
Jan 3, 2016 — [Xe] 4f^14 5d^10 The atomic number of Au is 79. Therefore, its configuration is: 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 4d^10 5s^2 5p^6 ...1 answer · [Xe]4f145d10 Explanation: The atomic number of Au is 79. Therefore, its configuration is: 1s22s22p63s23p63d104s24p64d105s25p64f145d106s1 or, [Xe]4f145d106s ...
Soaring Annual Switchover. 17 December, 2021 by salespodder. Looking for inspiration for your annual bookend get-together? Then here's another idea from the field. Huge engineering conglomerate BAE Systems. With their paws across everything from the biggest of aerospace tickets, through mega-defence spend to software.
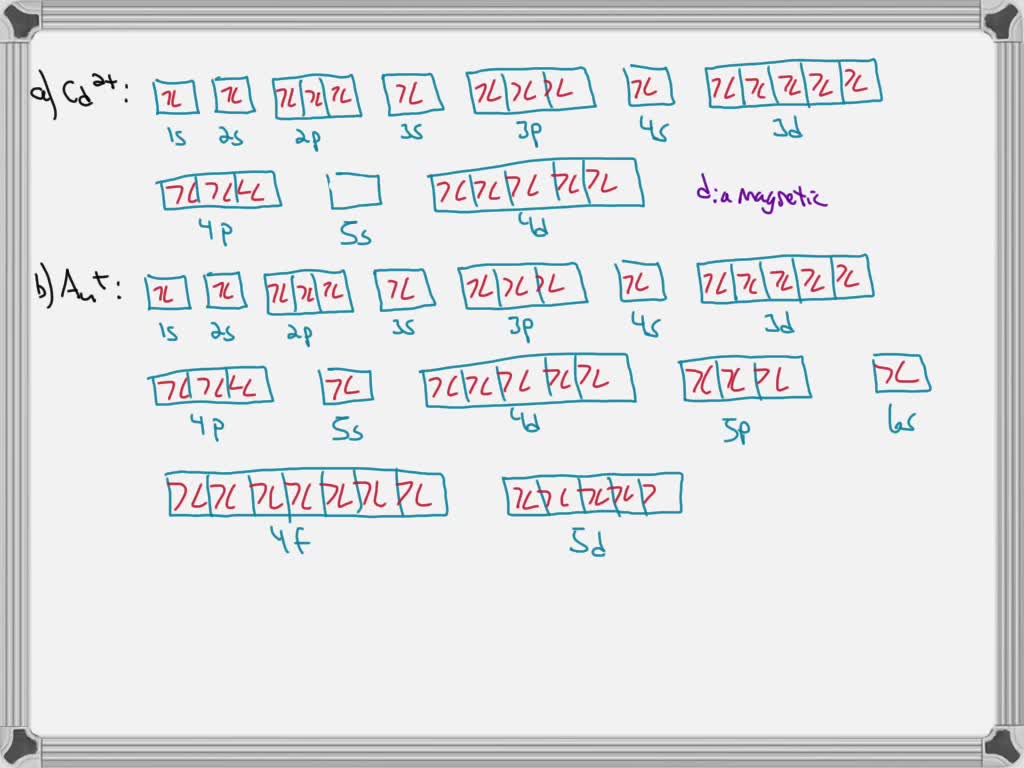
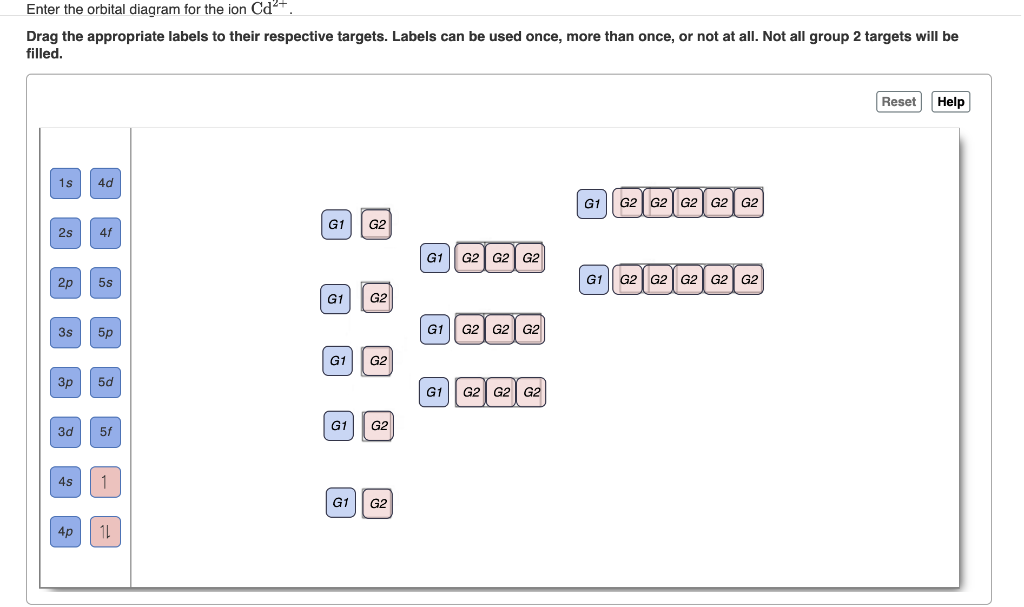
Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic a cd2 b au c mo3 d zr2 2















0 Response to "38 write orbital diagram for au+."
Post a Comment