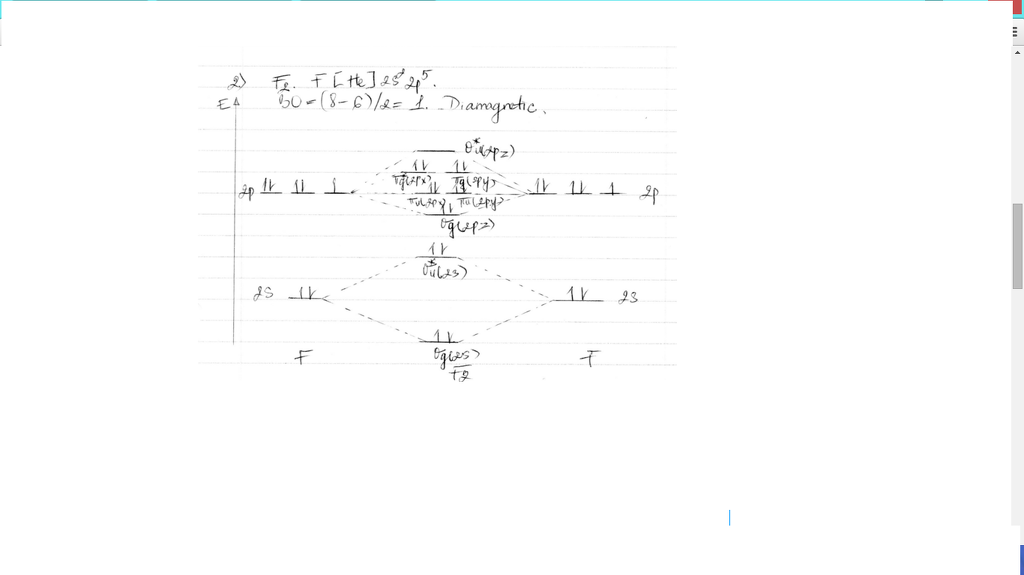
34 f2+ molecular orbital diagram
Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds. For the ion F2+:a) Draw the molecular orbital diagram.b) Calculate the bond order.c) Would this ion exist?d) Write the electron configuration of the ion.————...
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
F2+ molecular orbital diagram
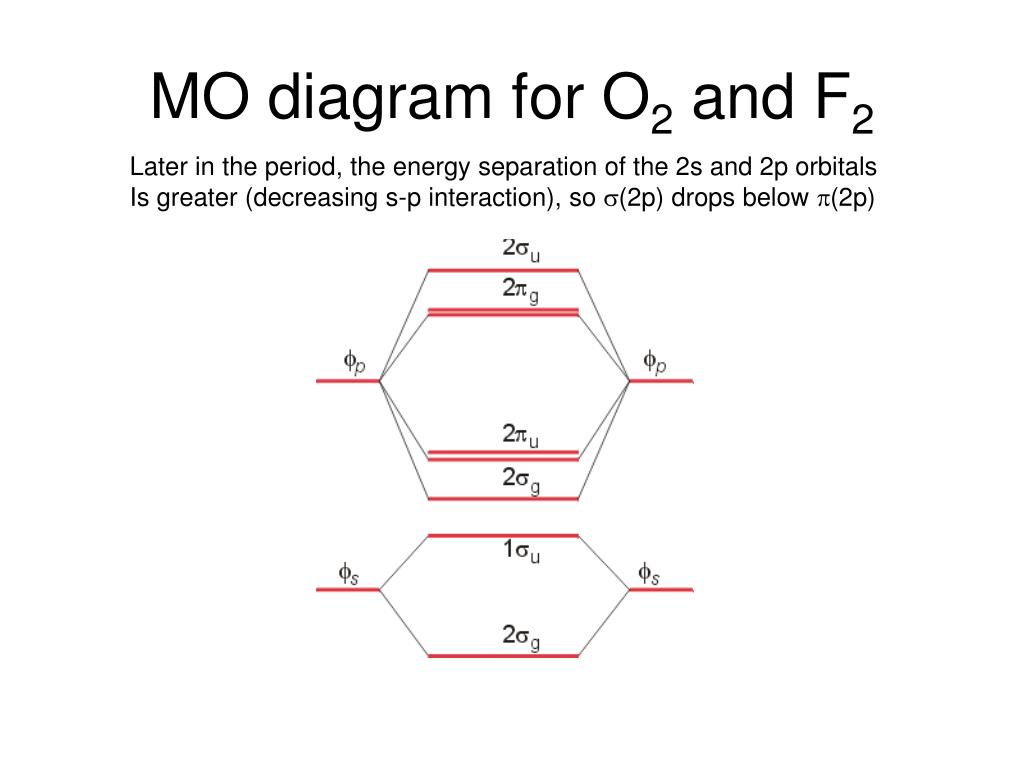
Problem: Use the molecular orbital diagram shown to determine which of the following is most stable.a.F22+b. Ne22+c. F22-d. O22+e. F2 Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways. Page 1. MO Diagrams for Elements Li2 through Ne2. (Don't memorize.) Li2 through N2. O2 through Ne2. Molecular orbitals were first introduced by Friedrich Hund and Robert S. Mulliken in 1927 and 1928. The linear combination of atomic orbitals or "LCAO" approximation for molecular orbitals was introduced in 1929 by Sir John Lennard-Jones. His ground-breaking paper showed how to derive the electronic structure of the fluorine and oxygen molecules from quantum principles.
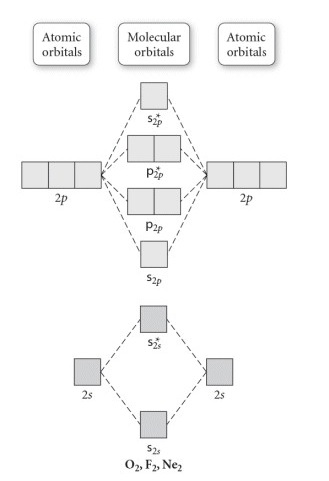
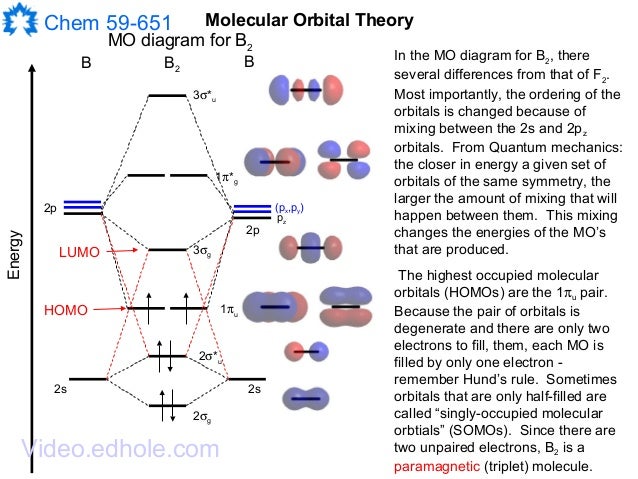
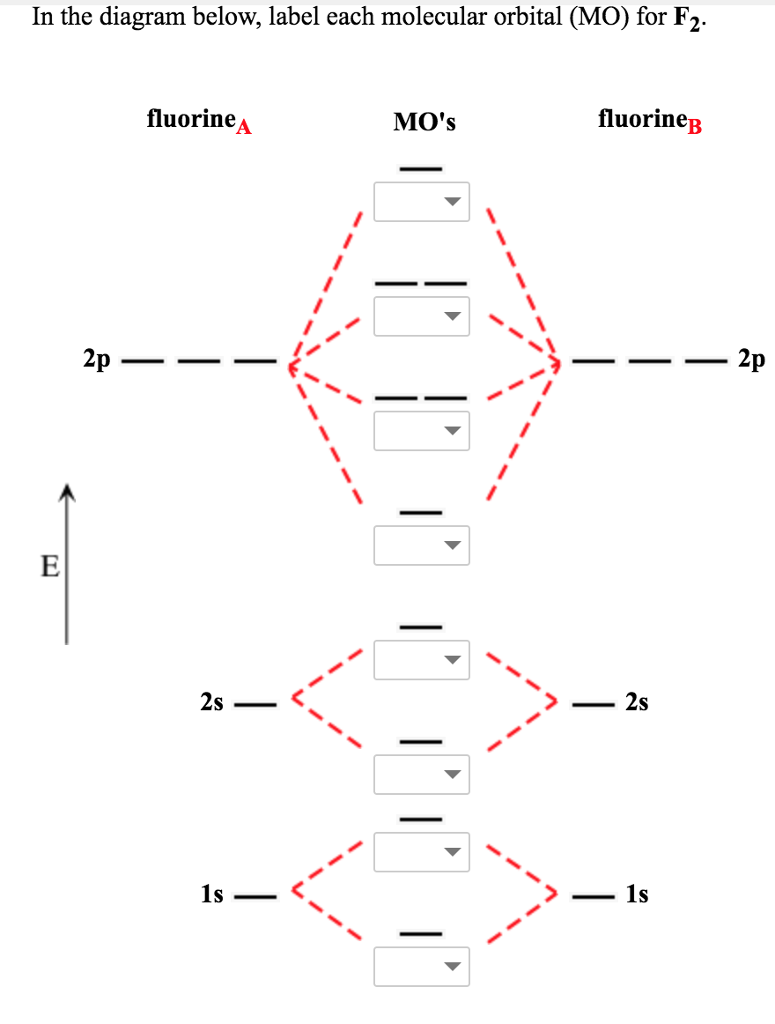
F2+ molecular orbital diagram. Arrange the following in order of decreasing stability. a blank molecular orbital diagram (part a 1 figure) has been provided to you. rank the fluorine species from most to least stable. to rank items as equivalent, overlap them. f2, f2+, f2- Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Molecular orbitals: Orbitals that span two or more atoms. These are constructed by overlapping atomic orbitals (AOs) which match in symmetry and size. In principle, To construct MO diagram of a any Molecule, first, set up Schrödinger wave equation for that molecule and then, solve it!!! The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is
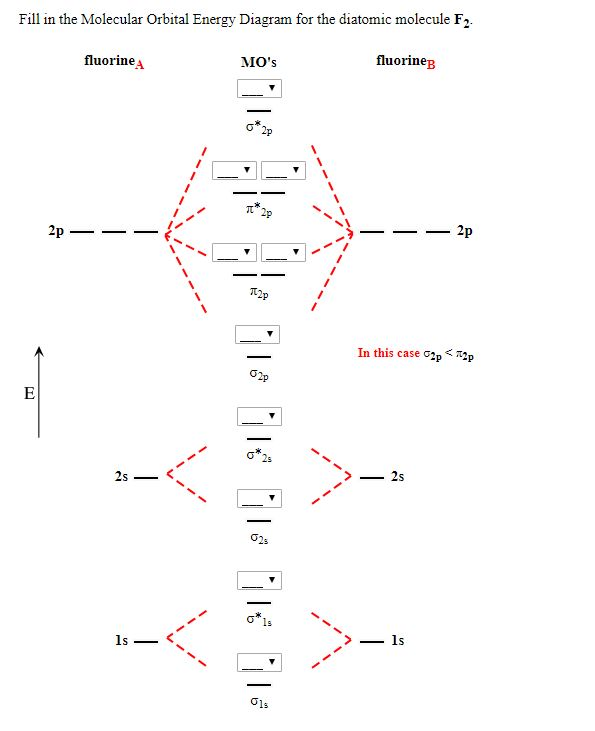
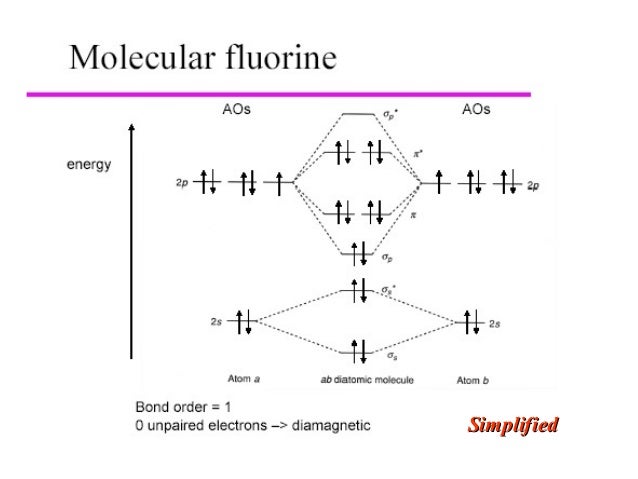
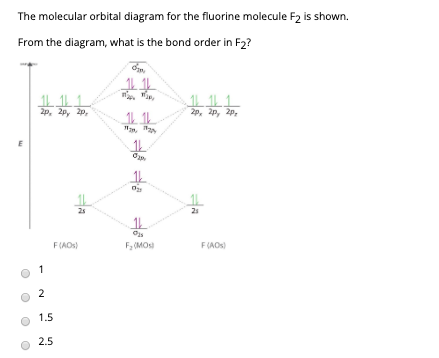
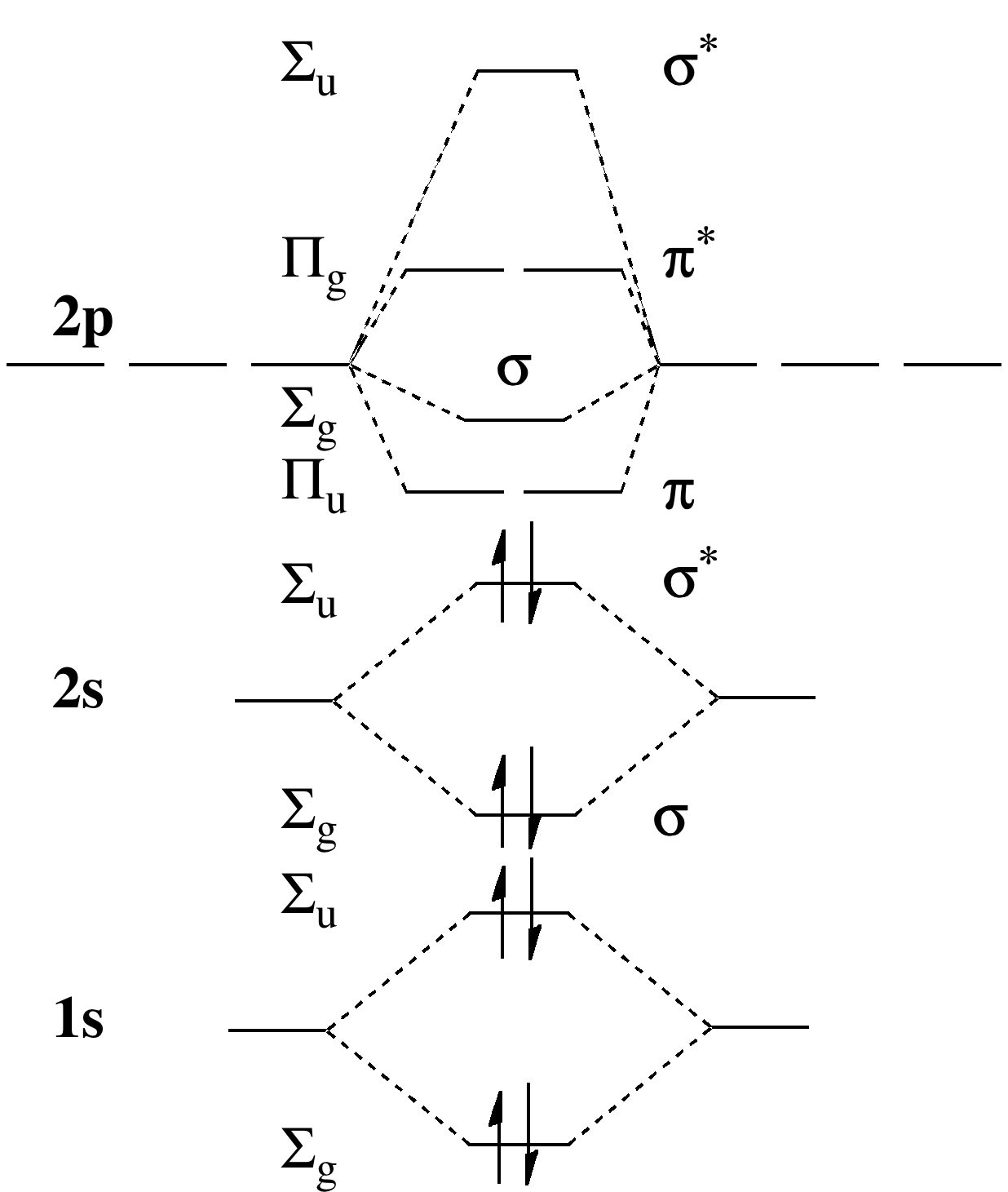
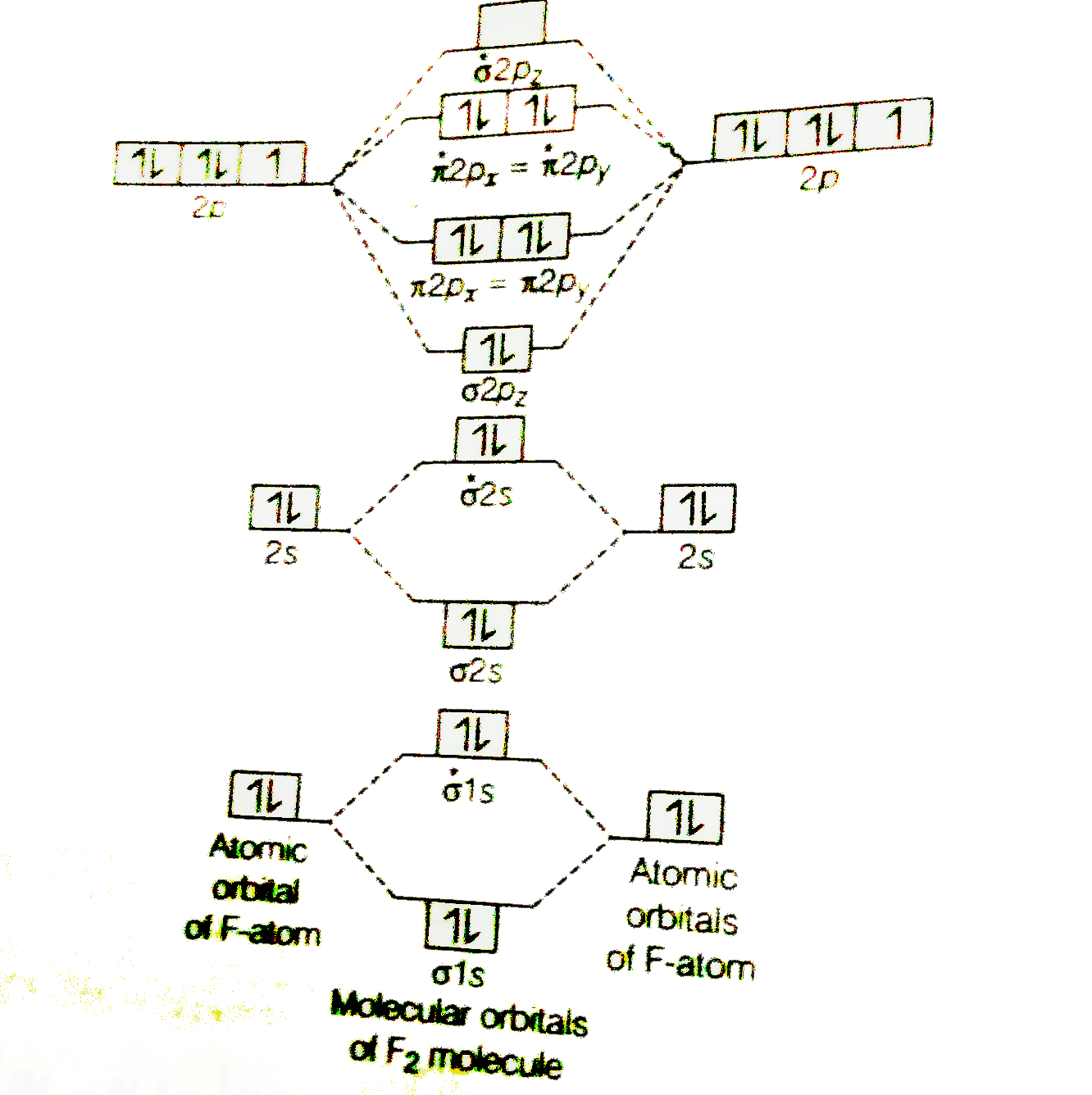
Below is the molecular orbital diagram for the fluorine molecule (F2) and images of several molecular orbitals that exist in this molecule. Fill the blanks below. Fatom F Fatom А ** tttt pfltit >pft t11 to f1 f1 att с D E F .t1 s stt 24 TL 1. a. The bond order for the F2 molecule is (enter a numeral). b. The F2 molecule is [paramagnetic Molecular orbital diagram f2. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for ... Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we’ll see that symmetry will help us treat larger molecules in A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
#sigma# molecular orbitals are singly-degenerate, and #pi# molecular orbitals are doubly-degenerate. #sigma# molecular orbitals, in principle, get more stabilized upon overlap than #pi# molecular orbitals do. For example, an #ns//ns# overlap for a homonuclear diatomic molecule gives rise to a partial MO diagram like this: When two fluorine atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.F2(2+) has a bond order of 2, so ... Q. Use the molecular orbital diagram shown to determine which of the following is most stable.a. F22+b.Ne22+c. F22-d. O22+e. F2 Q ... Our tutors rated the difficulty of Determine the bond order for F2, F2+ and F2 -. Which molecul... as high difficulty. Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ...

Chanelle Case Borden, Ph.D., a postdoctoral fellow in the National Cancer Institute's Experimental Immunology Branch, pipetting DNA samples into a tube for polymerase chain reaction, or PCR, a laboratory technique used to make multiple copies of a segment of DNA.
The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is
F2 Molecular Orbital (MO) Diagram As per molecular orbital (MO) theory, all the constituent atoms in a molecule contribute to the formation of molecular orbitals. These MOs are a linear combination of the atomic orbitals. Thus, the electrons in a molecule are not individually assigned to atomic orbitals but to molecular orbitals.
Click here👆to get an answer to your question ️ 37. Draw molecular orbital diagram for F2 molecule. Also, give its electronic configuration, bond order and magnetic property. 138. Solve the following:
Draw a molecular orbital diagram of ${N_2}$ or ${O_2}$ with magnetic behavior and bond order. Verified. 82.1k+ views. Hint: Generally the molecular orbital diagrams are used to understand the bonding of a diatomic molecule. You should know that molecular orbital diagrams are used to deduce magnetic properties of a molecule; they also help us to ...
When we make the molecular orbital energy level diagram of f2 molecule then, we will get this configuration: 1σs 2, 1σ*s 2, 2σs 2, 2σ* 2, σ2pz 2, π2p x2, π2p y2, πp x * 2, π2p y * 2. From this electronic configuration, we can see that there are a total of ten bonding molecular orbitals and eight antibonding molecular orbitals.
Draw molecular orbital diagram for F 2 molecule. Also, gives its electronic configuration, bond order and magnetic property. Hint: The Molecular Orbital Theory (MOT) explains the formation of the molecule in a better way than Valence Bond Theory (VBT). The bond order calculations are feasible using MOT and so is the description of electronic ...
The molecular orbital diagrams for molecules and ions are drawn from the order of increasing energies shown in the molecular orbital configuration. Always remember that the number of molecular orbitals formed must be equal to the number of atomic orbitals that were combined in the molecule.
The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is
Energy level diagram for Molecular orbitals. May 25, By Mrs Shilpi Nagpal 9 . It is paramagnetic in nature. 6)Li2. Molecular orbital energy level of Li2.Molecular orbitals of Li 2, Be 2, to F 2 The molecular orbital theory (MO) has been introduced for the diatomic hydrogen molecules.
A) F2; B) F2^2+ C) Ne2^2+ D) O2^2+ E) F2^2-2) Use molecular orbital diagrams to determine which of the following are paramagnetic. A) O2^2-B) Ne2^2+ C) O2^2+ D) F2^2+ E) None of the above are paramagnetic; 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic.
Formation of Molecular Orbitals. An atomic orbital is an electron wave; the waves of the two atomic orbitals may be in phase or out of phase. Suppose Ψ A and Ψ B represent the amplitude of the electron wave of the atomic orbitals of the two atoms A and B. Case 1: When the two waves are in phase so that they add up and amplitude of the wave is ...
[ê³ ë“±í•™êµ ê³ ê¸‰ 화학] ë™í•µ ì´ì›ìž 분ìžì˜ ê²°í•©ê³¼ ìƒìžê¸°ì„±(paramagnetic), ë°˜ìžê¸°ì„± ...
Molecular orbitals were first introduced by Friedrich Hund and Robert S. Mulliken in 1927 and 1928. The linear combination of atomic orbitals or "LCAO" approximation for molecular orbitals was introduced in 1929 by Sir John Lennard-Jones. His ground-breaking paper showed how to derive the electronic structure of the fluorine and oxygen molecules from quantum principles.
Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways. Page 1. MO Diagrams for Elements Li2 through Ne2. (Don't memorize.) Li2 through N2. O2 through Ne2.
Problem: Use the molecular orbital diagram shown to determine which of the following is most stable.a.F22+b. Ne22+c. F22-d. O22+e. F2














0 Response to "34 f2+ molecular orbital diagram"
Post a Comment