39 transition states energy diagram
ΔΔG‡ is the difference in the transition state energies for each process: ... during the 1980s, as methods of auxiliary mediated asymmetric synthesis advanced, "ee" spawned the term "de" (diastereomeric excess). The idea was that ... Energy diagram and reaction scheme for a kinetic resolution. The second diagram where the bonds are half-made and half-broken is called the transition state, and it is at this point that the energy of the system is at its maximum. This is what is at the top of the activation energy barrier. But the transition state is entirely unstable.
Sn2 Energy Diagram. Fig Transition state and energy diagram of an S N 2 reaction: Chloroform hydrolysis. They represent an energy maximum on the reaction coordinate. SN2 Reaction follows second order rate kinetics. It forms a product via one transition state. Transition state is the state at which it posses. It starts with the kinetics of SN2 ...
Transition states energy diagram
The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from a particular state to a lower state, it is losing energy. Transition state is the highest point (or points) on the reaction coordinate diagram. Those are the “peaks” or the “hills” in the picture. A more strict definition is that a transition state is a molecular entity that has a lifetime no longer than a vibration that exhibits some structural characteristics of both the reactants and the products. dissipate energy especially at electronic energy states higher than the first excited state. While this transition is slow, it is an allowed transition with the electron staying in the same multiplicity manifold. Fluorescence is most often observed between the first excited electron
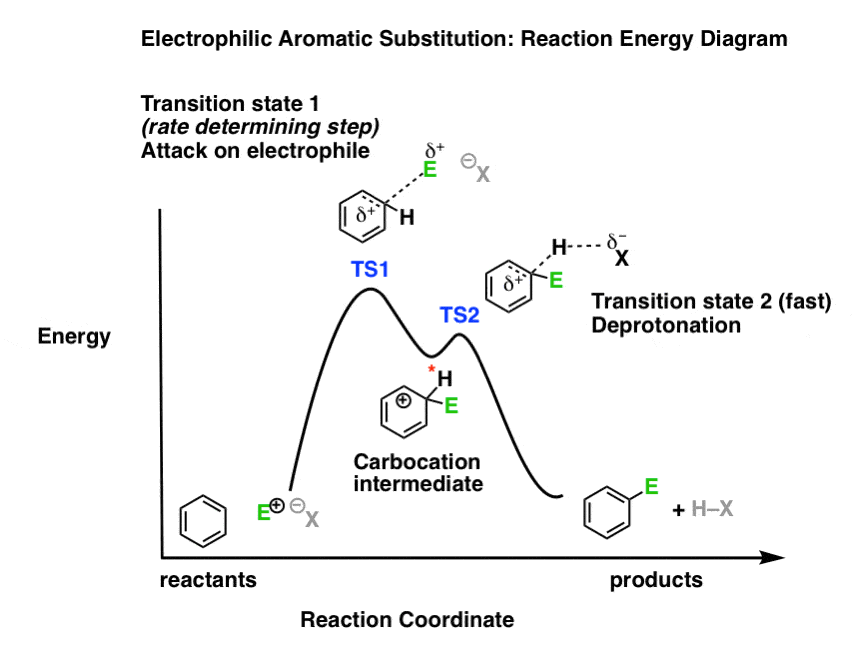
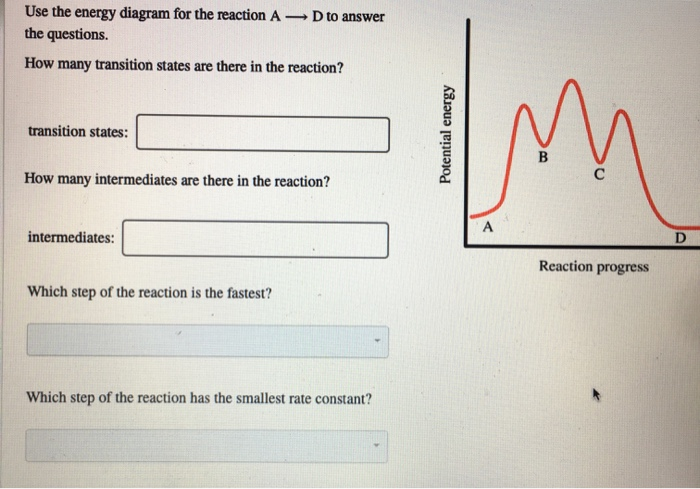
Transition states energy diagram. The free-energy diagram is shown in Figure 1.3. For multistep reactions, the slowest step is called rate-determiningstep (or rate-limiting step ). The reaction rate of the overall reaction is equal to the rate of rate-determining step. In the example shown in Figure 1.3, there are two transition states. Nov 21, 2010 · The energy difference between any two adjacent levels gets smaller as n increases, which results in the higher energy levels getting very close and crowded together just below n = ∞ . The ionization energy of an atom is the energy required to remove the electron completely from the atom.(transition from ground state n = 0 to infinity n = ∞ ). Use the energy diagram for the reaction A D to answer the questions. How many transition states are there in the reaction? transition states: Potential energy How many intermediates are there in the reaction? intermediates: Reaction progress Which step of the reaction is the fastest? Which step of the reaction has the smallest rate constant? a transition state. KEYWORDS: Reaction, Energy , Rate, Cation INTRODUCTION The easiest way to understand the difference between a transition state and an intermediate is to use what is commonly called a reaction (energy) diagram, like the one below. methylpropane with iodide, we know that the rate carbocation.
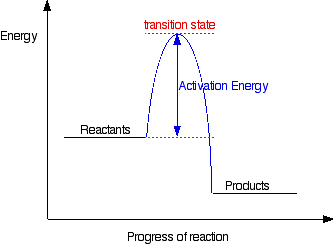
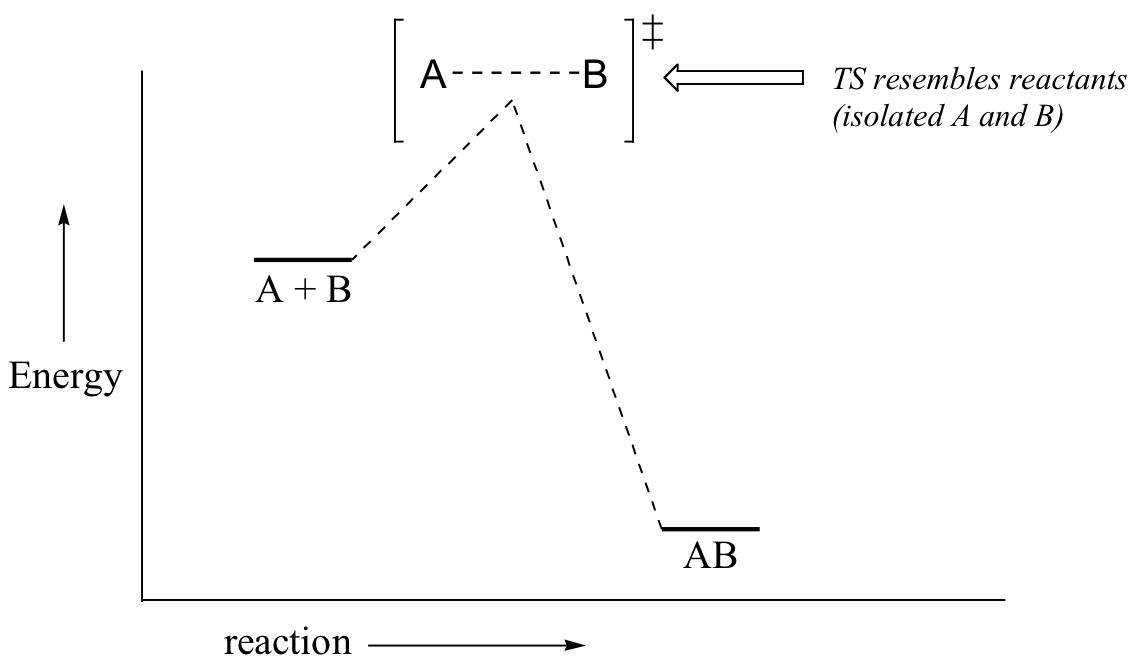
with reference to the diagram. Case 1: the beginning state is higher than the ending state – In this case Hammond’s postulate tells us that the TS ≠ will more closely resemble the beginning state. The energy of the TS≠ must be closer to the beginning state. There’s just no other way to draw the diagram. We call this TS≠ “early” since the structure of the transition state has ... That's the energy it takes for the reaction to occur. So it's from the reactants to the highest point on the curve in the transition state. So up here we have the transition state. And so the difference between the reactants and the transition state, as in 1, this difference, is what we call the activation energy. You might see that as E sub a. Both of the triplet state transitions are diagrammed on the right-hand side of the Jablonski energy profile illustrated in Figure 1. The low probability of intersystem crossing arises from the fact that molecules must first undergo spin conversion to produce unpaired electrons, an unfavorable process. Transition state theory (TST), also called activated complex theory, is often introduced in general chemistry courses when discussing kinetics. A reaction energy diagram is used to follow the progress of the reaction from reactants through a transition state to products (see figure 1). The reaction energy diagram plots the
In this video, we will go over how to draw an energy diagram for an organic reaction. Specifically, we will show the intermediates, transition states, and en... A transition state is a chemical species which has only fleeting existence and represents an energy maxima on reaction coordination diagram . While an intermediate lies in depression on potential energy curve . Therefore actual lifetime of an intermediate depends on the depth of the depression. This is part 3 of a four part series in the Energy Diagram Module. Stay tuned for Part 4! Click on the following links to see earlier parts: Part 1. Part 2. Sometimes reactions are more complex than simply a transition state (Graph 3), which would represent a single step in the reaction mechanism. Transition State in Energy Diagram Transition State in Energy Diagram Definition An energy diagram refers to the plot which shows the reactants' relative potential energies, its transition states, as well as the products progression of the reaction with time. It is a plot between potential energy and reaction progress or time.
A transition state is a very short-lived configuration of atoms at a local energy maximum in a reaction-energy diagram (aka reaction coordinate). A transition state has partial bonds, an extremely short lifetime (measured in femtoseconds), and cannot be isolated. Tip: Don’t confuse a transition state with an intermediate.
Transition state theory. Transition state theory ( TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes. TST is used primarily to understand qualitatively how chemical reactions take place.
The baseline in the Tanabe-Sugano diagram represents the lowest energy or ground term state. The d 2 case (not many examples documented). The electronic spectrum of the V 3+ ion, where V(III) is doped into alumina (Al 2 O 3 ), shows three major peaks with frequencies of: ν1=17400 cm -1 , ν2=25400 cm -1 and ν3=34500 cm -1 .
The state transition diagram as shown in Figure 8.6 illustrates the active and quiescent states that are supported by the logic and the paths between these states. The state transition diagram also illustrates the states and transitions of the communication protocol between the recipe phase and the equipment phase.
The energy levels agree with the earlier Bohr model, and agree with experiment within a small fraction of an electron volt. If you look at the hydrogen energy levels at extremely high resolution, you do find evidence of some other small effects on the energy. The 2p level is split into a pair of lines by the spin-orbit effect.
The energy of an atom can not vary continuously. It is quantified. The energy level diagram is used to represent the energy states available in each atom. When an electron is in an energy state, it emits nor absorbs radiation. A photon is emitted or absorbed when an electron transitions from one energy state to another.
Photons involved in transitions may have energy of various ranges in the electromagnetic spectrum, such as X-ray, ultraviolet, visible light, infrared, or microwave radiation, depending on the type of transition. In a very general way, energy level differences between electronic states are larger, differences between vibrational levels are ...
The active intermediate is shown in transition state at the top of the energy barrier. A class of reactions that also goes through a transition state is the S N2 reaction. A. The Transition State . We shall first consider S N 2 reactions [Substitution, Nucleophilic, 2nd order] because many of these reactions can be described by transition state theory. A Nucleophile is a substance (species ...
The energy changes for the above reaction can be represented in the energy diagram shown in Fig. 7.1. S N 2 is a single-step reaction, so the diagram has only one curve. The products CH 3 OH and Br - are in lower energy than the reactants CH 3 Br and OH - , indicates that the overall reaction is exothermic and the products are more stable.
The saddle point represents the highest energy point lying on the reaction coordinate connecting the reactant and product; this is known as the transition state. A reaction coordinate diagram may also have one or more transient intermediates which are shown by high energy wells connected via a transition state peak.
The transition states of such reactions are punctuated with reactive intermediates, which are represented as local minima on the energy diagrams. Reactive intermediates are products of bonds breaking and cannot be isolated for prolonged periods of time. Some of the most common reactive intermediates in organic chemistry are carbon ions or radicals.
Fig. 6-1. Energy level diagram of some of the excited states of the 12C nucleus. The angular momentum (J), parity (P), and isospin (T) quantum numbers of the states are indicated on the left using the notation J P. P and n respectively at the top of the diagram indicate the separation energies for a proton and a neutron.
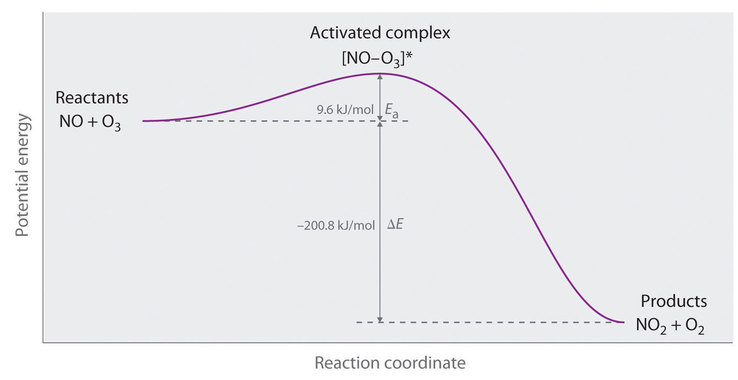
Energy diagrams are quite useful in illustrating these changes on a continuous basis as the reaction proceeds. Terms such as "activation energy" (Ea), "transition state" (*), and "enthalpy change" are easy to define by referring to a graph such as Figure 1.
An energy level diagram is a diagram that shows the energies of the reactants, the transition state (s) and the products of the reaction as the reaction proceeds ( called ‘extent of the reaction’ below) The transition state is a stage during the reaction at which chemical bonds are partially broken and formed

3 Schematic presentation of the potential energy of reactants A, B,... | Download Scientific Diagram
Dec 22, 2021 · The low GWP of such a system makes it an appealing addition to the suite of low-carbon technologies which will drive the energy transition. With this study illustrating the potential environmental advantages for solar-driven hydrogen production from biomass, further studies are recommended to assess the hydrogen production cost through this method.
REACTION RATES, TRANSITION STATES, AND MECHANISMS ... Reaction path diagrams, as represented above, are a two dimensional slice of a multidimensional energy surface for the reaction. Conventionally, they are a plot of energy (in the rigorous case, free energy, G) versus the reaction coordinate. 6. Reaction Mechanism. The mechanism of a reaction is a stepwise description of how reactants are ...
Energy Energy Diagrams 6 • Transition state ‡: - An unstable species of maximum energy formed during the course of a reaction. - A maximum on an energy diagram. • Activation Energy, ∆G‡: The difference in Gibbs free energy between reactants and a transition state. - If ∆G‡ is large, few collisions occur with sufficient
This particular intermediate configuration of atoms or molecules with the maximum value of potential energy is called activated complex and the state is defined as transition state. I m a g e w i l l b e U p l o a d e d S o o n Now the diagram above shows the transition state of a chemical reaction taking place.
On the far left of the diagram are the reactant species and on the far right are the product species. Transition State The transition state is the high energy point between two minima along the reaction coordinate. Each step in a mechanism will have a transition state.
dissipate energy especially at electronic energy states higher than the first excited state. While this transition is slow, it is an allowed transition with the electron staying in the same multiplicity manifold. Fluorescence is most often observed between the first excited electron
Transition state is the highest point (or points) on the reaction coordinate diagram. Those are the “peaks” or the “hills” in the picture. A more strict definition is that a transition state is a molecular entity that has a lifetime no longer than a vibration that exhibits some structural characteristics of both the reactants and the products.
The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from a particular state to a lower state, it is losing energy.




















0 Response to "39 transition states energy diagram"
Post a Comment