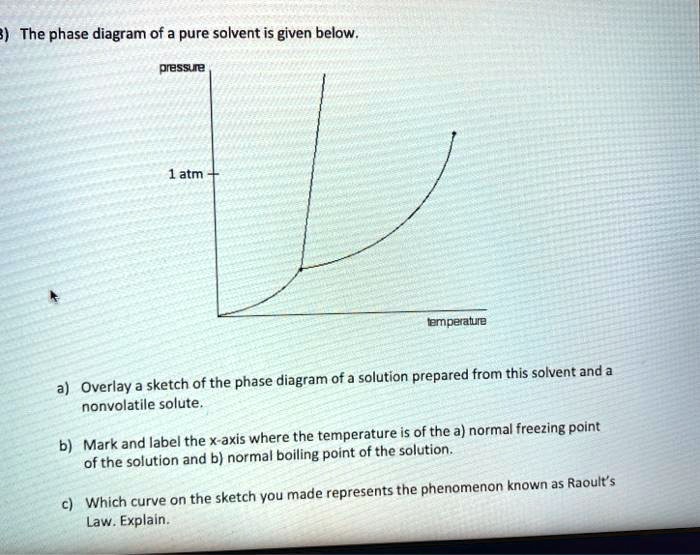
35 label the phase diagram of pure solvent and a solution
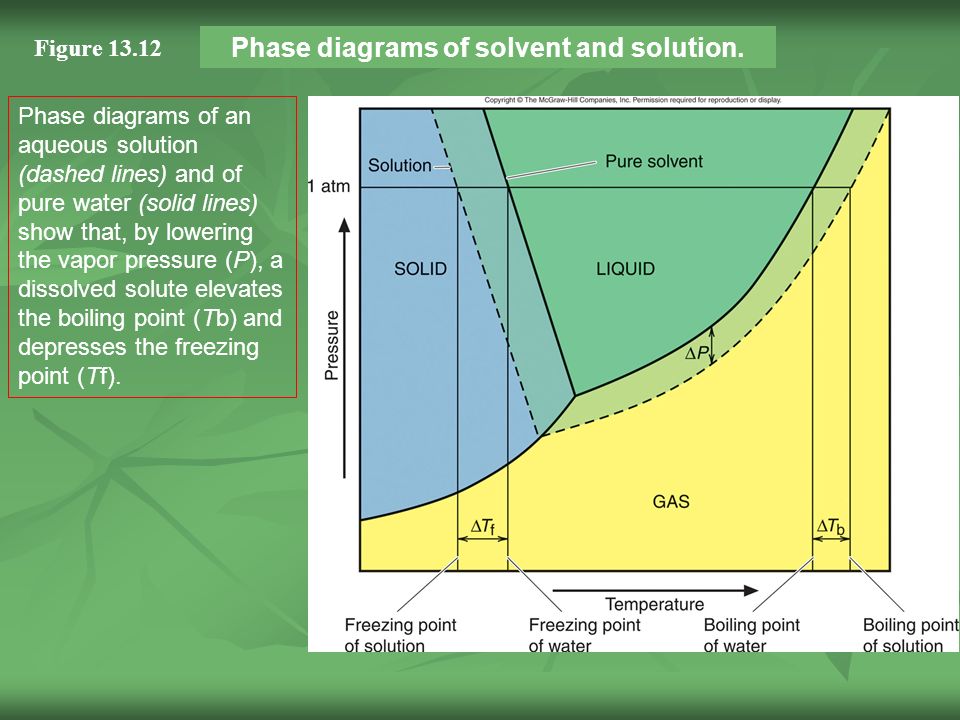
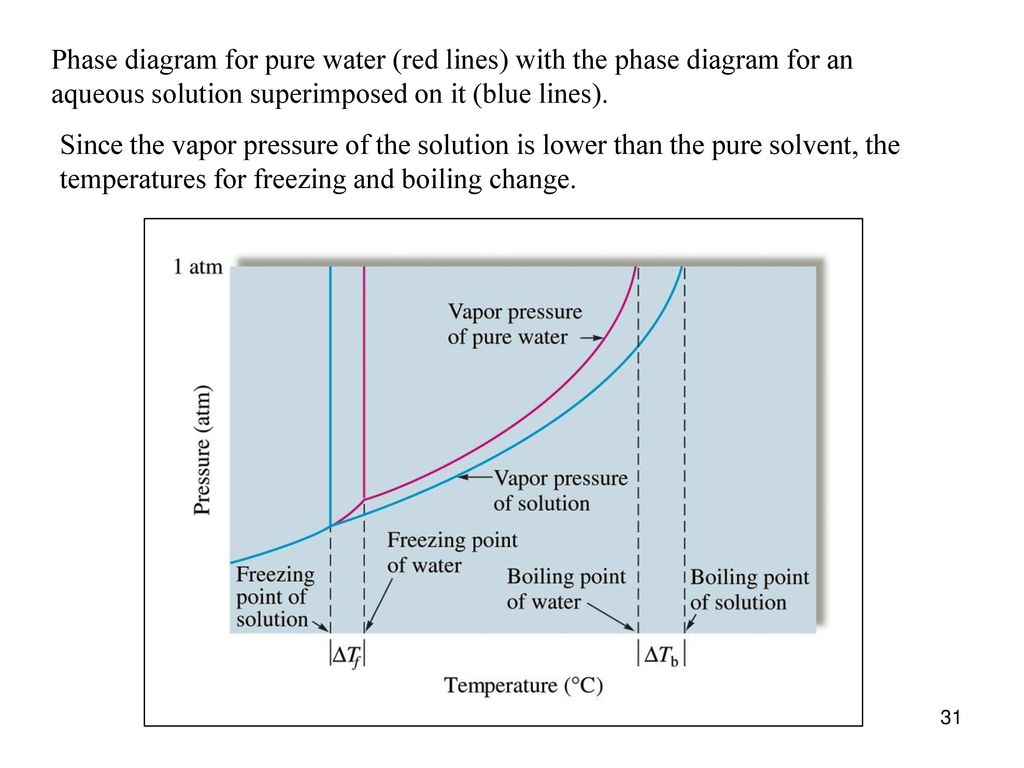
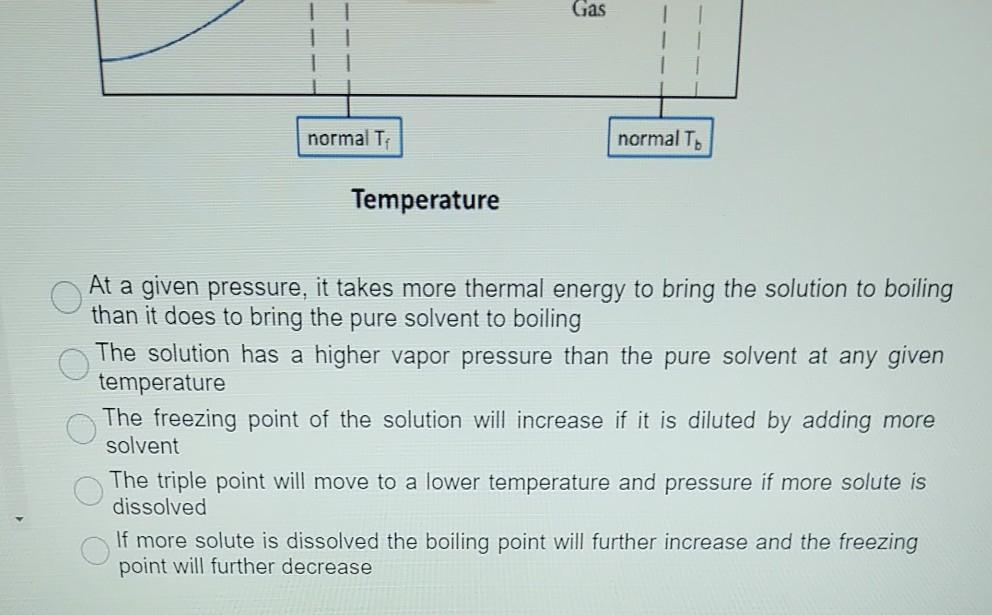
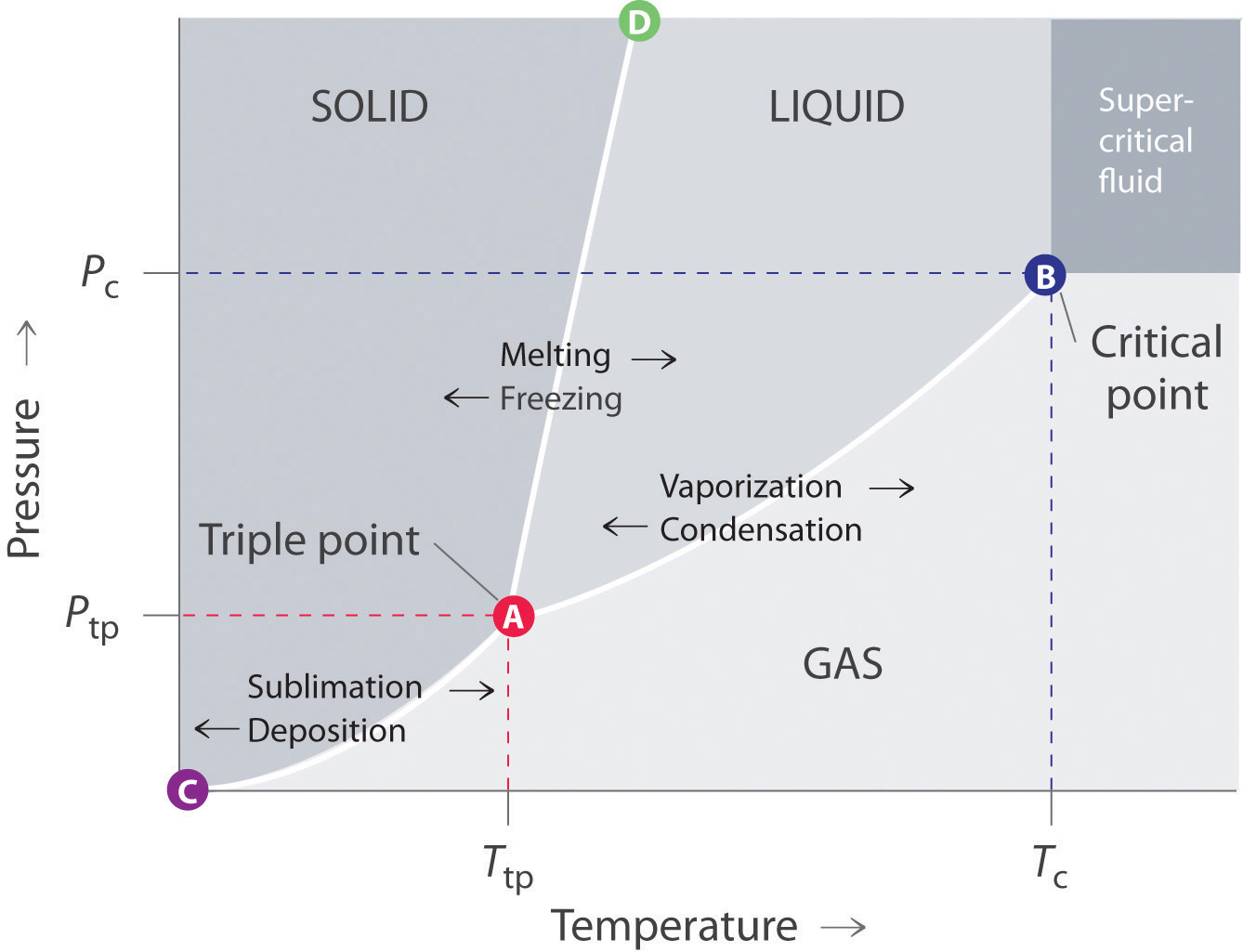
The figure shows two phase diagrams, one for a pure liquid ... Consider the following general phase diagram: Now, consider the pure substance, whose phase diagram was represented by the black curves. The normal freezing point of the pure substance at constant pressure is indicated by B, and its reduced normal freezing point (due to addition of nonvolatile solute to the pure liquid substance) is A, since Tdarr leftwards. Label The Phase Diagram Of Pure Solvent And A Solution ... Phase diagrams of pure water and an aqueous solution of a nonvolatile solute. Pure a and pure b are also considered to be α and β phases respectively. It has b atom as the solute component. A terminal phase or terminal solution is one that exists in the extremes of concentration 0 and 100 of the phase diagram.
At roughly what pressure and temperature w ... - Clutch Prep Q. Label the diagram of pure solvent and a solution. Q. Consider this phase diagram for carbon. Which phases are present at the lower triple point?a) diamondb) graphitec) gasd) liquidWhich phase is stable...
Label the phase diagram of pure solvent and a solution
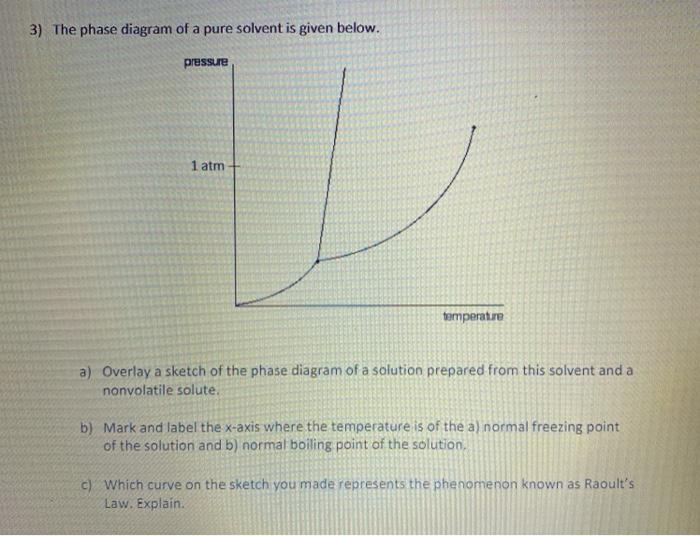
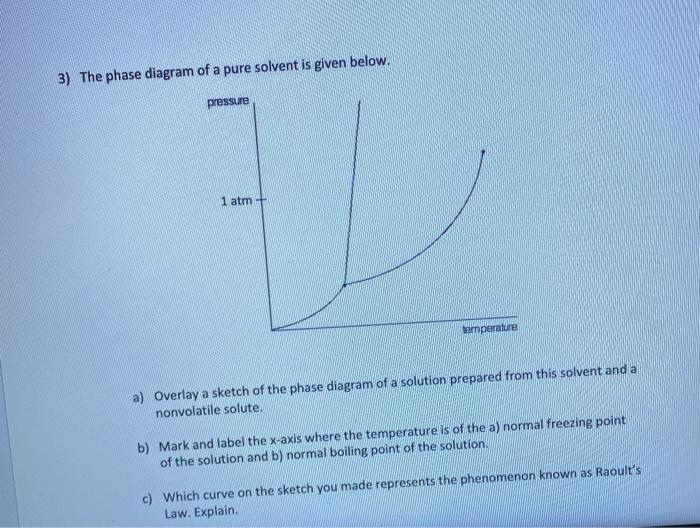
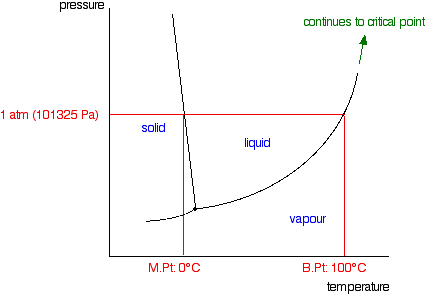
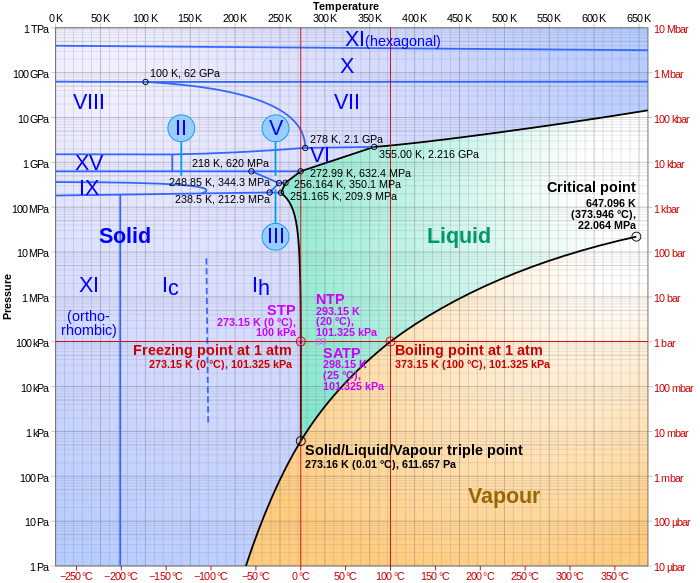
PDF Chapter Phase Diagrams - Uttar Pradesh Textile Technology ... • 281 Chapter 9 Phase Diagrams Liquid (Water) Pressure (atm) Vapor (Steam) 1,000 100 10 1.0 0.1 0.01 0.001 20 0 20 40 60 80 100 120 Solid (Ice) Temperature (°C) T he graph below is the phase diagram for pure H PDF Binary Solid-Liquid Phase Diagram Introduction Expt. 5: Binary Phase Diagram CHEM 366 V-3 Combination1,2,3 of equations (2), (3), (6), (7) and (10) leads to the equation T≅TA+ RTA 2 ΔHA lnXA (11) or T≅TA+ RTA 2 ΔHA ln(1−XB) (12) or T≅TA- RTA 2 ΔHA lnXB (13a) T≅TB- RTB 2 ΔHB lnXA (13b) where T and TA are freezing points (K) of the mixture II and pure A, R is 8.314 J/K mole, ΔHA is the molar enthalpy of fusion of A and XB ... PDF Colligative Properties AP Set II - Raleigh Charter High School The phase diagram for a pure substance is shown above. Use this diagram and your knowledge about changes of ... Use the data above to draw a phase diagram for argon. Label the axes and label the regions in which the solid, ... phase, solid argon must be the denser phase. (K) 5. (a) pure solvent solution 0 Time 0 5 10 15 20
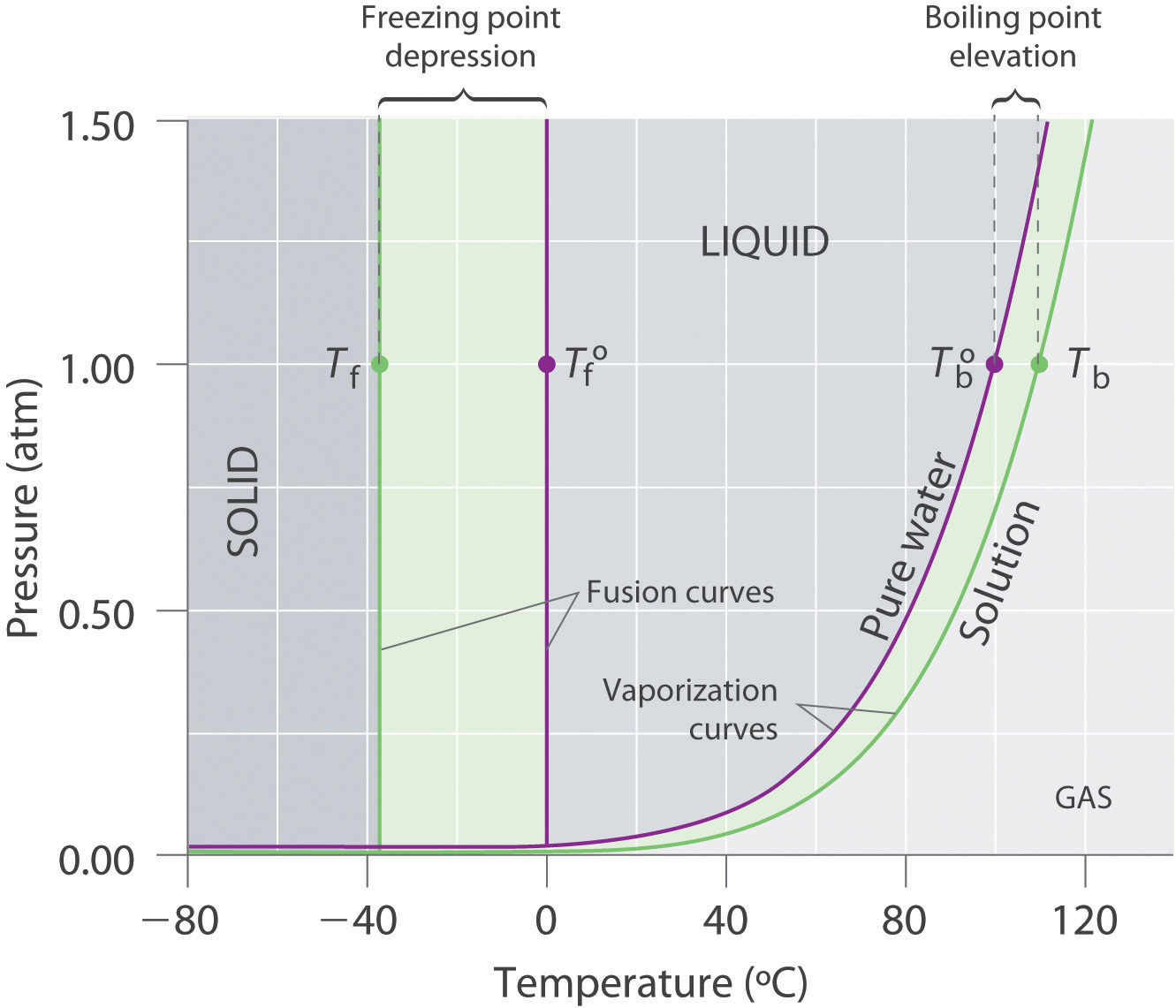
Label the phase diagram of pure solvent and a solution. Solid-liquid Phase Diagrams: Salt Solution The phase diagram for sodium chloride solution. What the phase diagram looks like. ... because adding a non-volatile solute to a solvent increases its boiling point. ... in this case, that's the pure ice crystals. On the other end, it hits the sloping line - this tells you the composition of the remaining salt solution. ... Solve this: â ‹Q64 The phase diagrams for the pure solvent ... The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below. The quantity indicated by 'L' in the figure is 1) ∆ P 2) ∆ T f 3) K p, m 4) K r, m Raveena Sharma, Meritnation Expert added an answer, on 22/4/18 The solution is as follows: 1. 'L' represents ∆ T b which is equal to K b .m. 10.4 Phase Diagrams - Chemistry A typical phase diagram for a pure substance is shown in Figure 1. Figure 1. The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2. SOLVED:Construct hypothetical phase diagram between 1000'â ... in Problem 36 were given a piece of a phase transition diagram where two solutions are said to have the same solvent but different Saul Utes. So you a has event off factor one, and so you be has event off factor off three. Now in part A. We have to match either Salyut eight or be to the red line in the graph or the blue line in the graph, and the main thing we have to focus on here is the fact ...
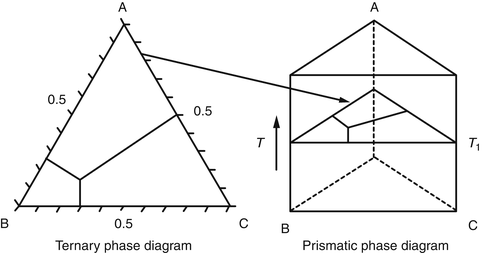
PDF Phase Diagrams, Solid Solutions, Phase Transformations Isomorphous Phase Diagrams Phase diagram Indicate phases as a function of Temp., Comp. and Pressure (under equilibrium condition) Binary phase diagram A phase diagram for a system with two components. Isomorphous phase diagram A phase diagram in which the components display unlimited solid solubility. Chapter 10: Solid Solutions and Phase ... Solved Label the phase diagram of pure solvent and a ... Label the phase diagram of pure solvent and a solution. Question: Label the phase diagram of pure solvent and a solution. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer. Who are the experts? Chemistry 1 Exam Flashcards - Quizlet Identify the solvent and solute in each of the solutions A) salt (NaCl) in water ... Also, assume that the engine coolant is pure ethylene glycol (HOCH2CH2OH), which is non-ionizing and non-volatile, and that the pressure remains constant at 1.00 atm. ... Label the phase diagram. A) Solid Phase B) Triple Point C) Sublimation Point/ Deposition Point PDF Chapter 9: Phase Diagrams - Florida International University - Solutions - solid solutions, single phase - Mixtures - more than one phase • Solubility Limit : Max concentration for which only a single phase solution occurs. Question: What is the solubility limit at 20°C? Answer: 65 wt% sugar . If Co < 65 wt% sugar: syrup If Co > 65 wt% sugar: syrup + sugar. 65 Sucrose/Water Phase Diagram Pure ...
Solved Label the phase diagram of pure solvent and a ... We review their content and use your feedback to keep the quality high. 100% (10 ratings) Transcribed image text: Label the phase diagram of pure solvent and a solution Freezing point of solution GAS Solution Pure solvent 1 atm Boiling pointFreezing point of of solvent solvent ??? 11 SOLID AT AP Boiling pointLIQUID of solution Temperature. Raoult's Law and Non-volatile Solutes - Blogger Raoult's Law and melting and boiling points The effect of Raoult's Law is that the saturated vapour pressure of a solution is going to be lower than that of the pure solvent at any particular temperature. That has important effects on the phase diagram of the solvent. The next diagram shows the phase diagram for pure water in the region around its normal melting and boiling points. Phase Diagrams - Chemistry Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram: (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase. Phase Diagram - SlideShare Phase Diagram 1. Chapter-5 PHASE AND PHASE EQUILIBRIUM Prepared By: PALLAV RADIA Asst prof. AITS, RAJKOT. 2. Introduction: One of the most important objective of engineering metallurgy is to determine properties of material. The properties of material is a function of the microstructure which depend on the overall composition and variable such as pressure and temperature. Hence to determine ...
PDF Phase Diagrams Phase Diagrams Revised: 1/27/16 3 The phase diagram in Figure 1 is for a pure compound. When a second compound is introduced to the system forming a homogeneous solution however, the phase diagram drastically changes. For example, the addition of a solute to a pure solvent (making a solution)
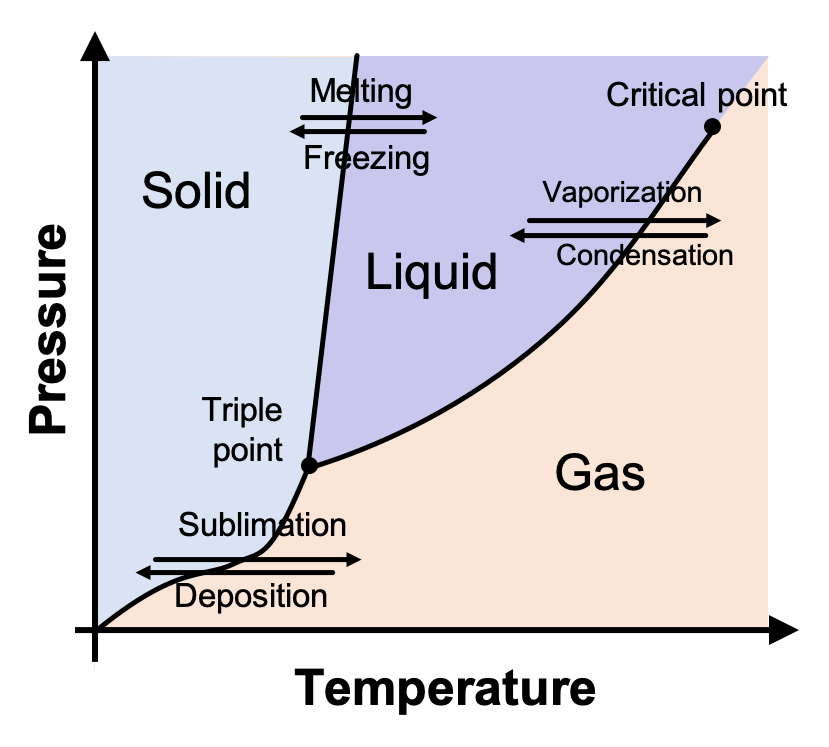
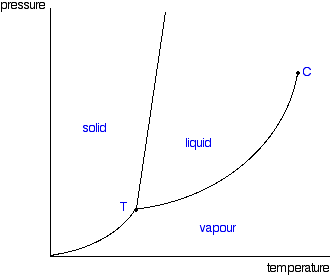
phase diagrams of pure substances - chemguide Phase diagrams. A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance.
Label the diagram of pure solvent and a so... | Clutch Prep Problem: Label the diagram of pure solvent and a solution. FREE Expert Solution Recall that a phase diagram shows the transition of matter between solid, liquid, and gas phases as temperature and pressure changes.
PDF Chapter 13: Solutions Phase Diagram for a solution compared to the pure solvent: Freezing Point Depression: T f = imK f Example 11: In making homemade ice cream, the freezing point of the surrounding ice-water salt slush solution is lowered by using 11.5% by mass sodium chloride solution which is observed to freeze at -8.02°C. The normal freezing point of water is
PDF Solutions a) a solution consists only of a single phase b ... Solutions 1. Circle the following that are true statements about solutions a) a solution consists only of a single phase b) a solution must have more than one component c) solutions must be liquid. 2. The component in the solution with the largest mode fraction is referred to as the (solvent, solute). Solvent 3.
Answered: The phase diagrams for a pure solvent… | bartleby The phase diagrams for a pure solvent and the solvent in a solution are shown. Identify the normal freezing (fpsolv) and boiling (bpsolv) points for the pure solvent and the normal freezing (fpsoln) and boiling (bpgoln) points of the solution at 1 atm. Assume the solute is nonvolatile and that the solid that freezes from solution is pure solvent. 1 atm Liquid Solid Answer Bank fpsolv bpsolv ...
The phase diagram for solvent and solutions is shown in ... >> The phase diagram for solvent and soluti. Question . The phase diagram for solvent and solutions is shown in the figure. What represents the normal boiling point of the solution? A. A. B. B. C. C. D. D. Hard. Open in App. Solution. Verified by Toppr. ... The phase diagram for a pure substance is shown above.
(Solved) - 1. For binary solid-liquid aquarium, show ... 1 Answer to 1. For binary solid-liquid aquarium, show rigorously that the "freezing out" of the solvent from a metastable supercoiled liquid phase results in an overall decrease in the Gibbs free energy. Remember that for a multicomponent system. 2. Using ideal solution models for a binary...
PDF Colligative Properties AP Set II - Raleigh Charter High School The phase diagram for a pure substance is shown above. Use this diagram and your knowledge about changes of ... Use the data above to draw a phase diagram for argon. Label the axes and label the regions in which the solid, ... phase, solid argon must be the denser phase. (K) 5. (a) pure solvent solution 0 Time 0 5 10 15 20
PDF Binary Solid-Liquid Phase Diagram Introduction Expt. 5: Binary Phase Diagram CHEM 366 V-3 Combination1,2,3 of equations (2), (3), (6), (7) and (10) leads to the equation T≅TA+ RTA 2 ΔHA lnXA (11) or T≅TA+ RTA 2 ΔHA ln(1−XB) (12) or T≅TA- RTA 2 ΔHA lnXB (13a) T≅TB- RTB 2 ΔHB lnXA (13b) where T and TA are freezing points (K) of the mixture II and pure A, R is 8.314 J/K mole, ΔHA is the molar enthalpy of fusion of A and XB ...
PDF Chapter Phase Diagrams - Uttar Pradesh Textile Technology ... • 281 Chapter 9 Phase Diagrams Liquid (Water) Pressure (atm) Vapor (Steam) 1,000 100 10 1.0 0.1 0.01 0.001 20 0 20 40 60 80 100 120 Solid (Ice) Temperature (°C) T he graph below is the phase diagram for pure H















0 Response to "35 label the phase diagram of pure solvent and a solution"
Post a Comment