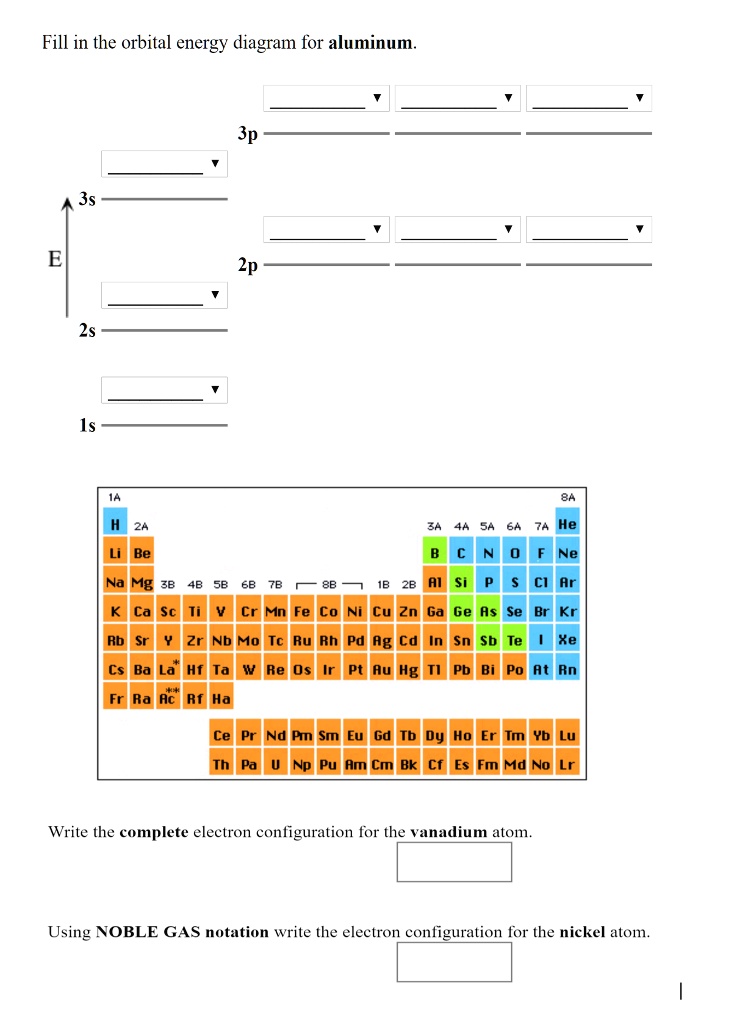
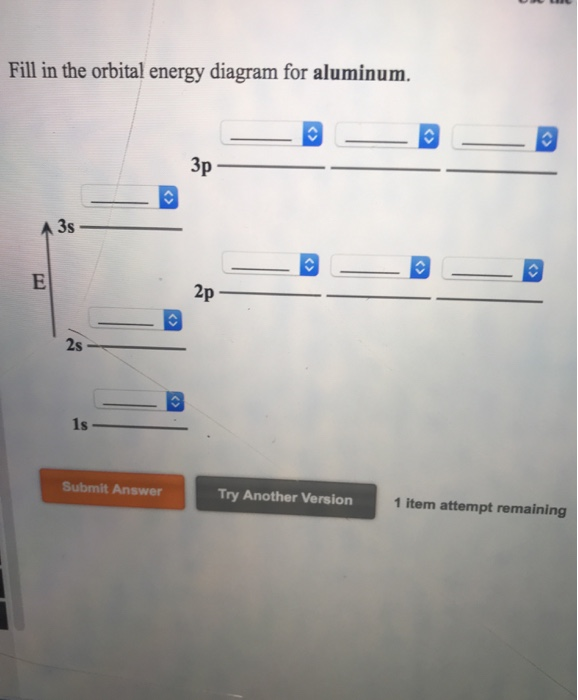
35 orbital diagram for aluminum
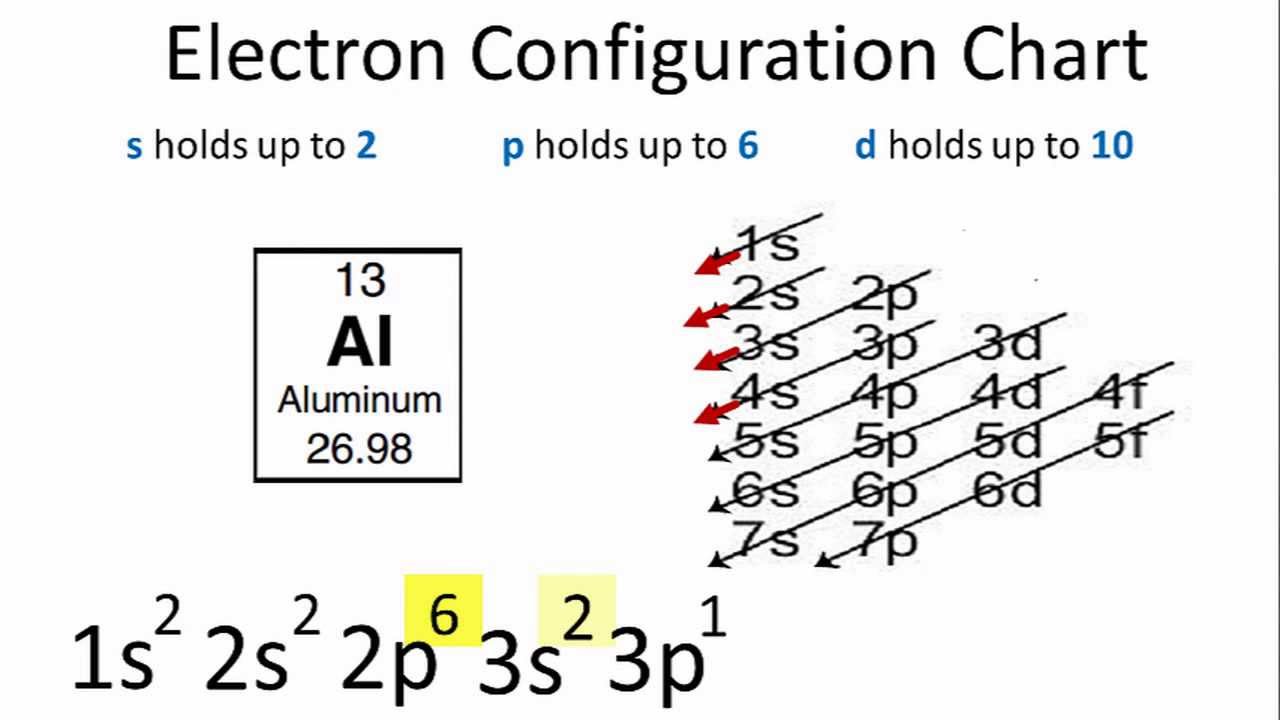
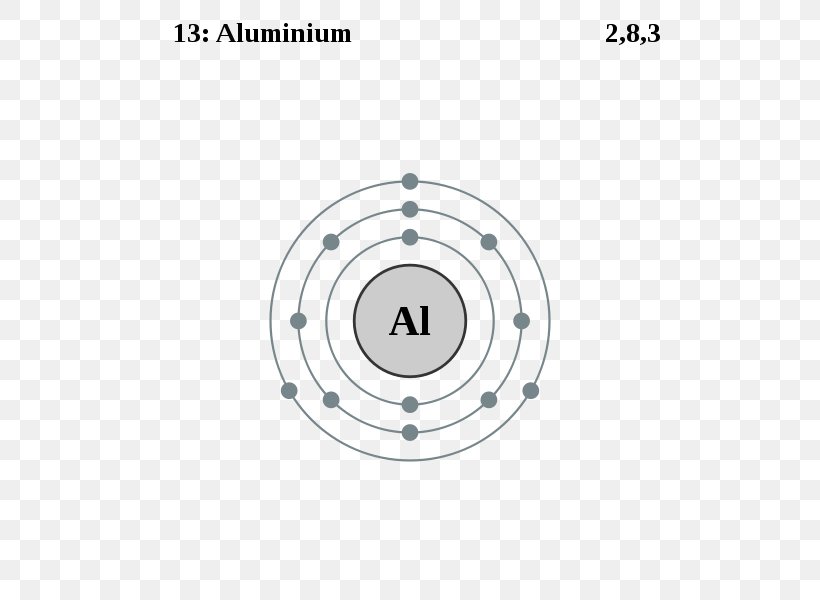
topblogtenz.com › sodium-bohr-modelSodium Bohr Model - How to draw Bohr diagram for Sodium(Na) atom Electron dot diagram of a Sodium atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Sodium, we got to know, it has only 1 valence electron. So, just represent the 1 valence electrons around the Sodium atom as a dot. Electron configuration for Aluminum (element 13). Orbital ... Melting point: 660.5 ℃. Density: 2.7 g/cm 3 . Electronic configuration of the Aluminum atom: 1s 2 2s 2 2p 6 3s 2 3p 1. Reduced electronic configuration Al: [Ne] 3s 2 3p 1. Below is the electronic diagram of the Aluminum atom Distribution of electrons over energy levels in the Al atom. 1-st level (K): 2. 2-st level (L): 8.
serc.carleton.edu › research_education › geochemElectron probe micro-analyzer (EPMA) - Techniques Sep 27, 2019 · Quantitative EPMA analysis is the most commonly used method for chemical analysis of geological materials at small scales. In most cases, EPMA is chosen in cases where individual phases need to be analyzed (e.g., igneous and metamorphic minerals), or where the material is of small size or valuable for other reasons (e.g., experimental run product, sedimentary cement, volcanic glass, matrix of ...

Orbital diagram for aluminum
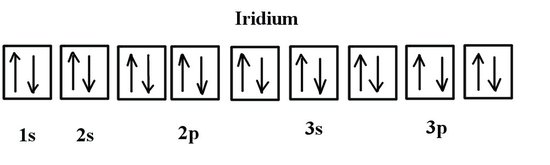
Aluminum - Basics The orbital diagram of aluminum helps show the specific "address" of each electron. Each arrow in the diagram represents a single electron (arrow pointing up if positive spin, down if negative spin). The numbers above the squares of the diagram represent the energy level, and the letters represent sub levels. Each box represents an orbital, also. What is the orbital diagram of aluminum? - Quora Answer: Aluminum (Al) has only atomic orbitals. As a 3rd row element, however, it has a complete Ne electron configuration. One way to denote this is to write it as the following: [Ne] 3s(2) 3p(1), The parentheses indicate the number of electrons in each orbital. The full electron shell (includ... Gadolinium(Gd) electron configuration and orbital diagram Gadolinium(Gd) is the 64th element in the periodic table and its symbol is 'Gd'. Gadolinium is a classified lanthanide element. This article gives an idea about the electron configuration of gadolinium and the orbital diagram, period and groups, valency and valence electrons of gadolinium, bond formation, compound formation, application of different principles.
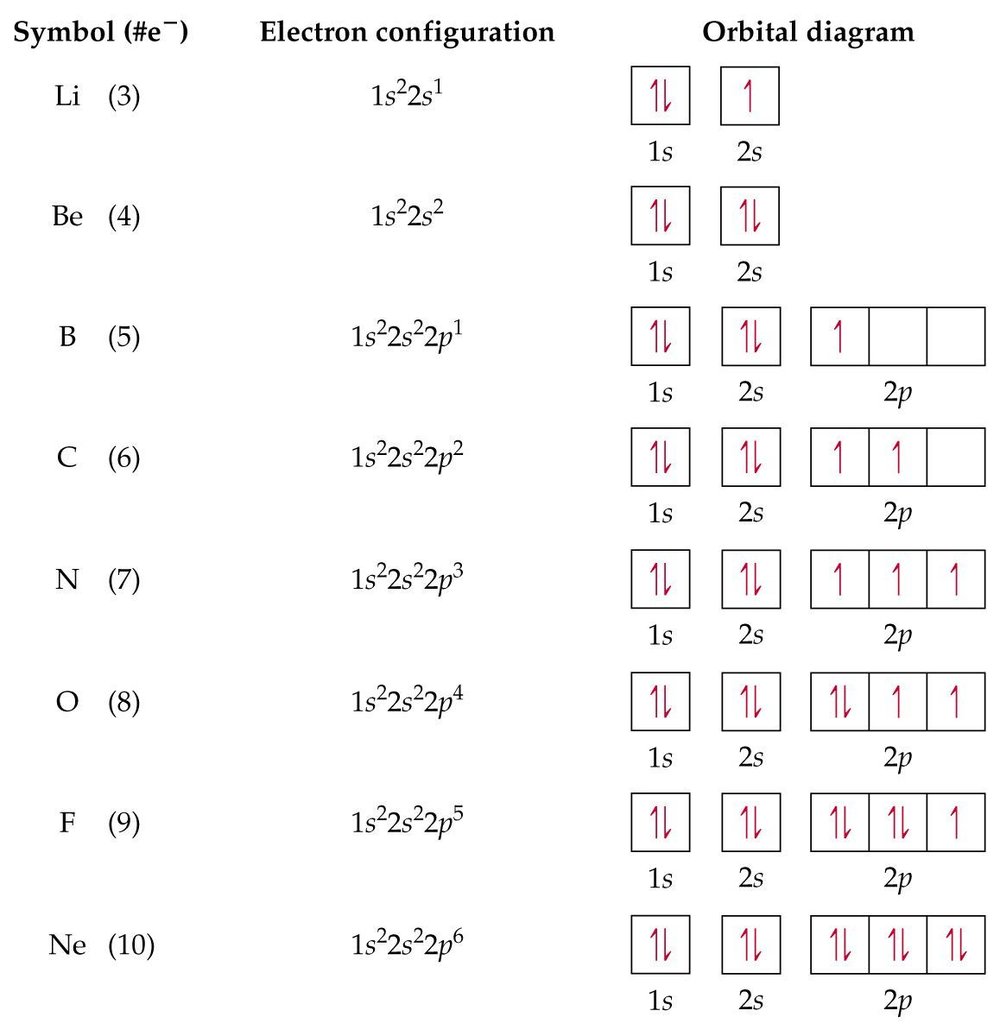
Orbital diagram for aluminum. valenceelectrons.com › nitrogen-electron-configurationNitrogen(N) electron configuration and orbital diagram Orbital Diagram for Nitrogen (N) Nitrogen(N) excited state electron configuration. Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of nitrogen is 1s 2 2s 2 2p 3. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z. Each sub-orbital can have a ... What is the electron configuration of aluminum and its ... What is an orbital diagram? An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to... your-online.ru › en › electronic-formulasElectron configuration for Molybdenum (element 42). Orbital ... The order of filling the orbitals with electrons in the Mo atom is an exception to the rule. Expected electronic configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 4 But in reality, one electron moves from the 5s orbital to the 4d orbital: Electron Configuration for Aluminium (Al) In writing the electron configuration for Aluminium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for aluminium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
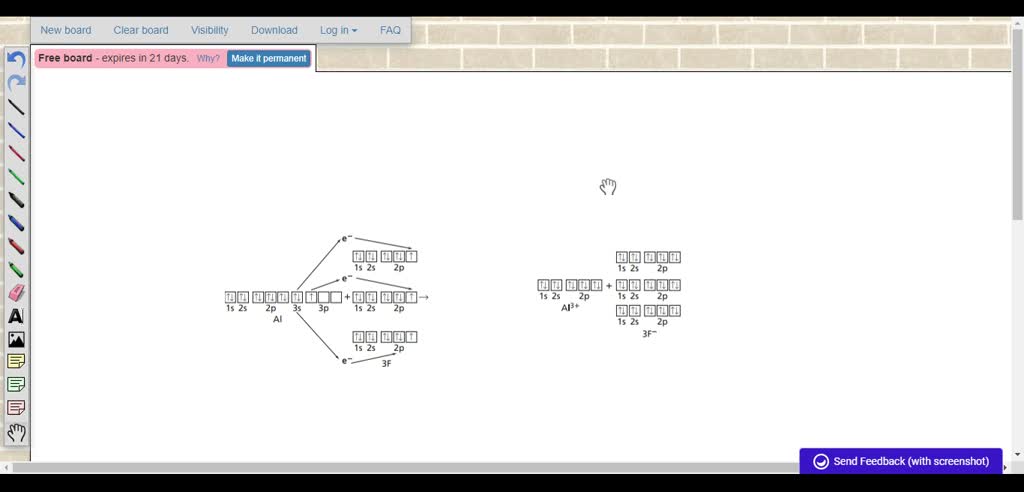
Aluminum Bohr Model - How to draw Bohr diagram for ... The Bohr model of Aluminum (Al) is drawn with three electron shells, the first shell contains 2 electrons, the second shell contains 8 electrons and the third shell contains 3 electrons. Aluminum is neutral and its atomic number is 13, hence, the number of protons and electrons available for its Bohr diagram is also 13. en.wikipedia.org › wiki › Space_debrisSpace debris - Wikipedia Orbital perturbations cause longitude drift of the inoperable spacecraft and precession of the orbital plane. Close approaches (within 50 meters) are estimated at one per year. [49] The collision debris pose less short-term risk than from an LEO collision, but the satellite would likely become inoperable. How many valence electrons does aluminum(Al) have? The electron configuration of aluminum ion(Al 3+) is 1s 2 2s 2 2p 6. The electron configuration of aluminum-ion shows that aluminum ions have only two shells and the last shell has eight electrons. The electron configuration shows that the aluminum ion(Al 3+) has acquired the electron configuration of neon. Aluminum(Al) electron configuration with orbital diagram Aluminum (Al) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. The first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. The 1s orbital is now filled with two electrons.
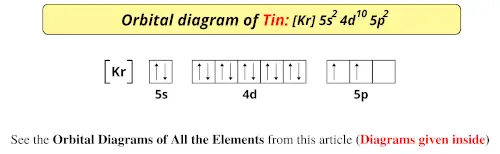
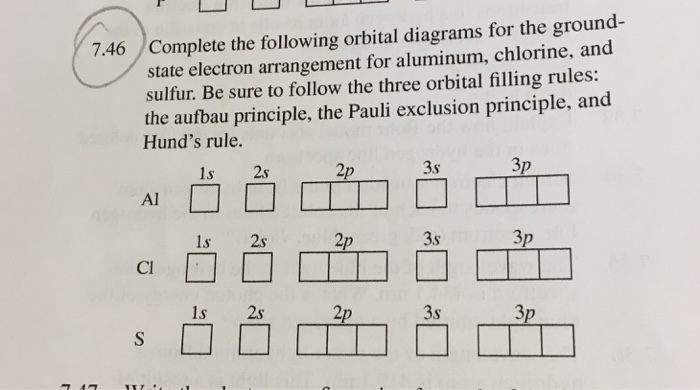
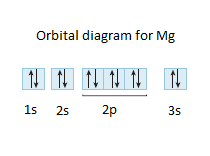
EOF periodictableguide.com › orbital-diagram-of-allOrbital Diagram of All Elements (Diagrams given Inside) Apr 10, 2021 · Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15: Orbital diagram of Phosphorus (P) 16: Orbital diagram of Sulfur ... Solved Please draw the orbital diagram for Aluminum. Based ... Please draw the orbital diagram for Aluminum. Based on your drawing, is aluminum attracted to a manget? Aslo, Draw the orbital diagram for Fe3+. Again, based on your orbital diagram for Fe3+, is this ion attracted to a magnet? Aluminum Orbital diagram, Electron configuration, and ... The Aluminum orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, the six electrons in 2p orbital, the two electrons in 3s orbital, and the remaining one in 3p orbital. Orbital diagram for a ground-state electron configuration of an Aluminum atom is shown below- What is the electron configuration of the Al3+ ion?
Chem4Kids.com: Aluminum: Orbital and Bonding Info Aluminum (Al) and phosphorus (P) can also bond. Aluminum happens to have three extra electrons. Luckily, every phosphorus atom is looking to gain three electrons. It's a perfect match! Something to notice though, look how they have a bond with six electrons. That bond is known as a triple bond. When a bond has two electrons it is a single bond.
Aluminum Bohr Diagram A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Jan 18, · Aluminum, Al Bohr Diagram.
topblogtenz.com › nitrogen-bohr-modelNitrogen Bohr Model - How to draw Bohr diagram for Nitrogen(N ... Electron dot diagram of a Nitrogen atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Nitrogen, we got to know, it has 5 valence electrons. So, just represent these 5 valence electrons around the Nitrogen atom as a dot.
How to Write the Orbital Diagram for Aluminum (Al) - YouTube To write the orbital diagram for the Aluminum atom (Al) first we need to write the electron configuration for just Al. To do that we need to find the number ...
PDF Electron Configurations and Orbital Diagrams key Write the electron configuration (full, and in core notation) for the following ions: 1.-1Br +3 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 2. Sr +2 8. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4s [Kr], [Ar] 3d 10 4s 2 4p 6 3. +2Se-2 9. 1s 2 2s2 2p6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 4.
Gadolinium(Gd) electron configuration and orbital diagram Gadolinium(Gd) is the 64th element in the periodic table and its symbol is 'Gd'. Gadolinium is a classified lanthanide element. This article gives an idea about the electron configuration of gadolinium and the orbital diagram, period and groups, valency and valence electrons of gadolinium, bond formation, compound formation, application of different principles.
What is the orbital diagram of aluminum? - Quora Answer: Aluminum (Al) has only atomic orbitals. As a 3rd row element, however, it has a complete Ne electron configuration. One way to denote this is to write it as the following: [Ne] 3s(2) 3p(1), The parentheses indicate the number of electrons in each orbital. The full electron shell (includ...
Aluminum - Basics The orbital diagram of aluminum helps show the specific "address" of each electron. Each arrow in the diagram represents a single electron (arrow pointing up if positive spin, down if negative spin). The numbers above the squares of the diagram represent the energy level, and the letters represent sub levels. Each box represents an orbital, also.



















0 Response to "35 orbital diagram for aluminum"
Post a Comment