36 delta g energy diagram
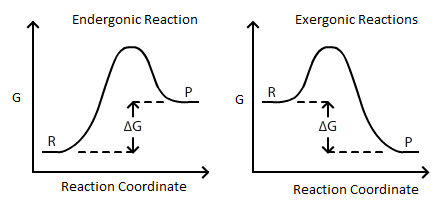
Energy Diagrams.pdf - Exergonic : < 0 Endergonic: > 0 ... Exergonic : < 0 Endergonic: > 0 Different from exothermic and endothermic which is enthalpy not overall reactions Delta G < 0 is spontaneous but does not imply a significant rate of reaction Reaction Coordinate Diagrams Low energy pathway Bigger Ea is slower reaction Delta G of reverse rxn is the same magnitude Transition State stays ... Free energy | Endergonic vs exergonic reactions (article ... Gibbs free energy and spontaneous reactions. Endergonic, exergonic, exothermic, and endothermic. Free energy. This is the currently selected item. Next lesson. ATP and reaction coupling. Sort by: Top Voted. Endergonic, exergonic, exothermic, and endothermic. Biology is brought to you with support from the Amgen Foundation.
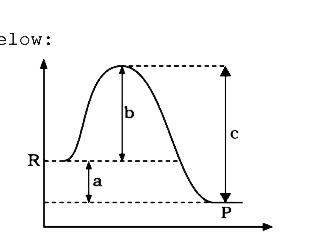
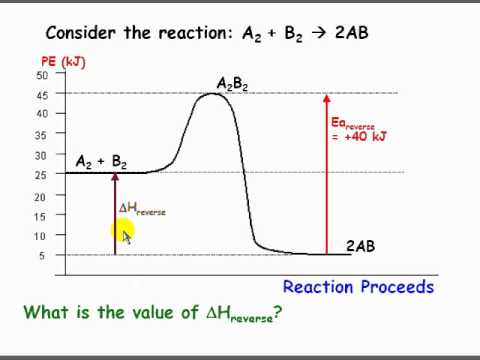
Solved Use the reaction energy diagram above to answer the ... Calculate the activation energy, Delta G for the step C to B kcal mol Calculate the overall energy change, Delta G degree, for the process B to A. kcal mol Which step is faster, B to A or B to C? Use the reaction energy diagram above to answer the following questions.
Delta g energy diagram
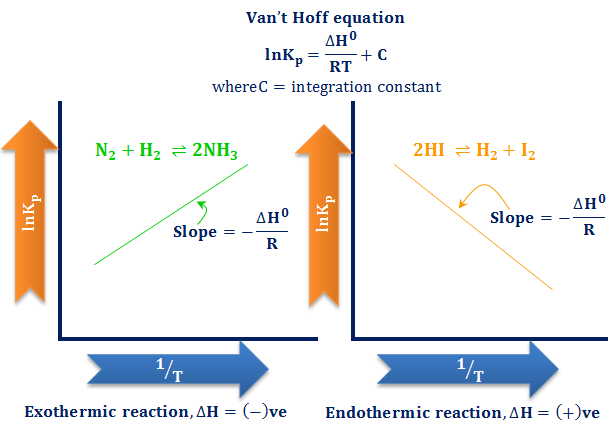
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... Gibbs Free Energy | ΔG = ΔH - TΔS - Chad's Prep® Finally, the change in Gibbs free energy is zero (ΔG=0) for a reaction that has reached equilibrium. These are summarized in the table below. ΔG = ΔH - TΔS The change in Gibbs free energy (ΔG) for a system depends upon the change in enthalpy (ΔH) and the change in entropy (ΔS) according to the following equation: ΔG = ΔH - TΔS ΔGo = ΔHo - TΔSo Ellingham diagram represents A Change of Delta G with ... The Ellingham diagram is a curve related to the value of the Gibbs free energy with temperature. Gibbs energy change is, $\Delta G=\Delta H-T\Delta S$ ---- (1) Where $\Delta H$ are the change in enthalpy and $\Delta S$ the change in entropy. From the above reaction, Gibbs free energy related to the equilibrium constant as,
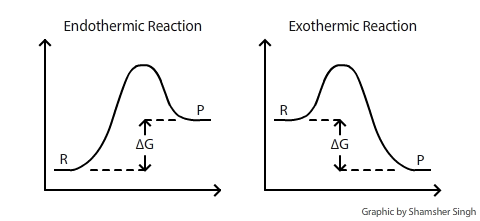
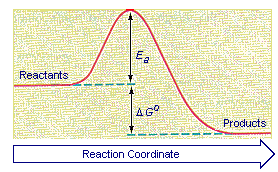
Delta g energy diagram. Potential, Kinetic, Free, and Activation Energy ... Every chemical reaction involves a change in free energy, called delta G (∆G). The change in free energy can be calculated for any system that undergoes a change, such as a chemical reaction. To calculate ∆G, subtract the amount of energy lost to entropy (denoted as ∆S) from the total energy change of the system. How to draw the potential energy diagram for this reaction ... Since heat is released for "C"_3"H"_8(g) + 5"O"_2(g) -> 3"CO"_2(g) + 4"H"_2"O"(g) + 2219.9" kJ", we say that DeltaH_(C)^@ = -"2219.9 kJ/mol propane". We approximate that this is the change in potential energy for the reactants going to the products. The above is for an endothermic reaction. A certain feature of combustion reactions suggests that you should draw the diagram DIFFERENTLY from ... Chapter 6, An Overview of Organic Reactions ... - Numerade Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the second step has a higher-energy transition state than the first. Label the parts of the diagram corresponding to reactant, product, intermediate, overall $\Delta G^{\dagger},$ and overall $\Delta G^{\circ}.$ Delta G Equation & the Equilibrium Constant | How to Find ... Reaction Diagram Displaying Delta G When Delta G is less than zero (dG < 0) as in the diagram above, the reaction is said to be spontaneous, which means the reaction will tend towards the products....
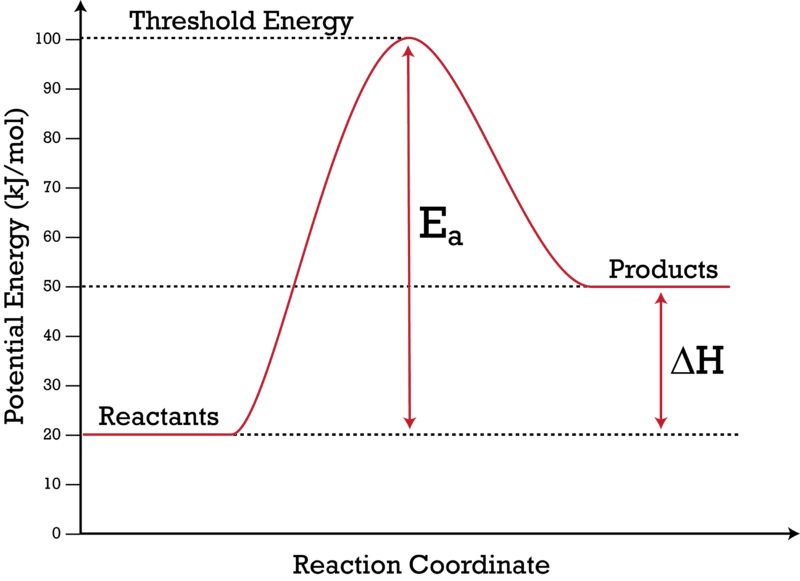
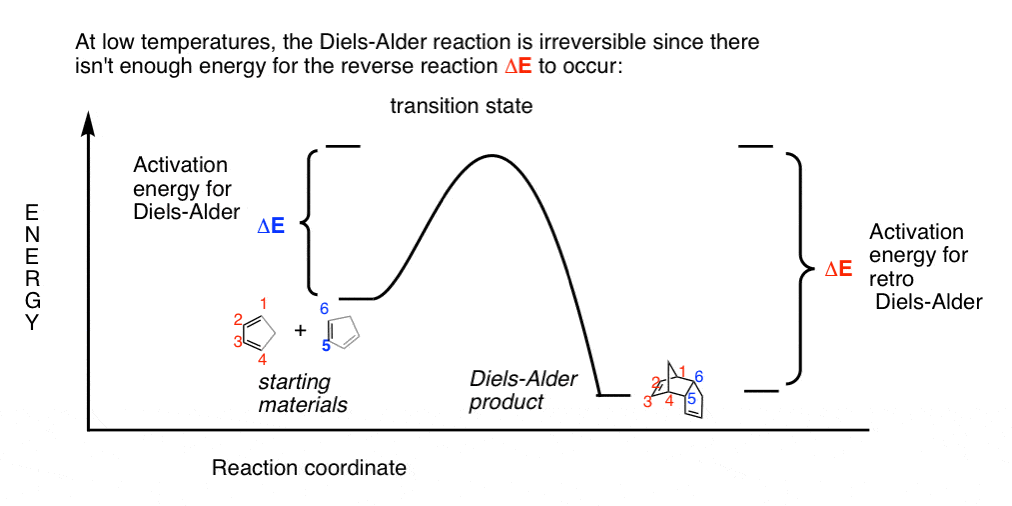
Using Potential Energy Diagrams.flv - YouTube Shows how a potential energy diagram can be used to determine activation energy and enthalpy change (Delta H) for forward and reverse reactions. Shows how a ... Work-Energy Theorem Problems and Solutions for High School 23.09.2021 · Problem (11): A $55-{\rm g}$ bullet is fired to a tree trunk. It moves at $575\,{\rm m/s}$ and penetrate the trunk a depth of $4.5\,{\rm cm}$. Find the average frictional forces that act on the bullet to stop it by applying work and energy considerations? Gibbs free energy introduction (video) - Khan Academy So just to phrase this again, the delta G, or change in Gibbs-free energy, reaction tells us very simply whether or not a reaction will occur. Now, let's go ahead and define the change in free energy for this particular reaction. ... which hopefully is more clear by this visual diagram, is that the change in free energy, which remember is equal ... How to calculate Delta H - Easy To Calculate Delta h in chemistry, also written as ∆H, refers to the change of enthalpy in a thermodynamic system caused by either absorption or emission of thermal energy. In simpler words, we may elaborate delta h as the total change in the system’s heat before and after a chemical reaction.
Physical Equilibria - Chemistry 302 The diagram you mostly find associated with different phases of a substance is the so-called "phase diagram". ... Thus the vapor pressure depends on the standard free energy of vaporization. \[\Delta G^\circ_{\rm vap} =\Delta H^\circ_{\rm vap} -T\Delta S^\circ_{\rm vap}\] Band structure of graphene, massless Dirac fermions as low ... Oct 08, 2020 · Graphene as the first truly two-dimensional crystal. The surprising experimental discovery of a two-dimensional (2D) allotrope of carbon, termed graphene, has ushered unforeseen avenues to explore transport and interactions of low-dimensional electron system, build quantum-coherent carbon-based nanoelectronic devices, and probe high-energy physics of "charged neutrinos" in table-top experiments. Star-Delta Transformer Connection Overview 07.11.2021 · The delta connected winding carries third harmonic current due to which potential of neutral point is stabilized. Some saving in cost of insulation is achieved if HV side is star connected. But in practice the HV side is normally connected in delta so that the three phase loads like motors and single phase loads like lighting loads can be supplied by LV side using … Potential Energy Diagrams - Kentchemistry.com A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values.
Using the Gibbs change, Δ G^o = + 63.3kJ , for the ... Given that Δ f G ∘ for C u (a q) 2 + and Z n (a q) 2 + are 65 kJ mol − 1 and 147.2 kJ mol − 1 respectively, then the standard Gibbs energy change for the above reaction is: Hard View solution
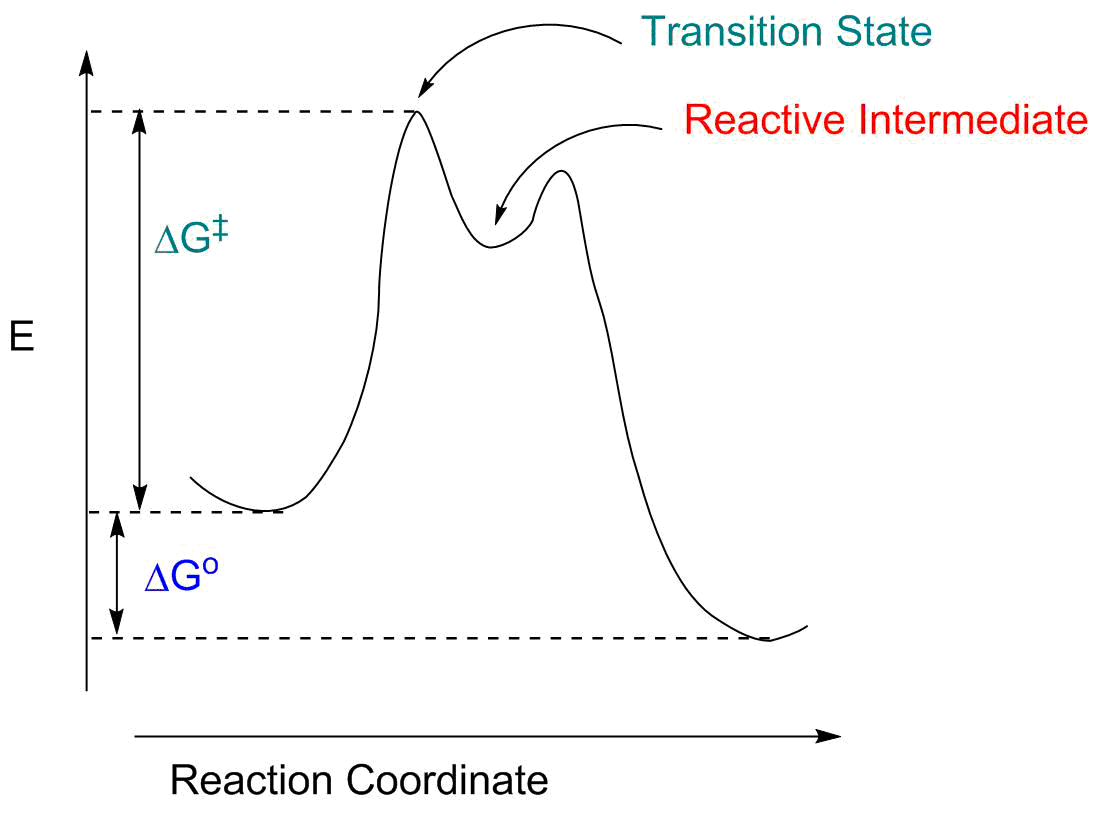
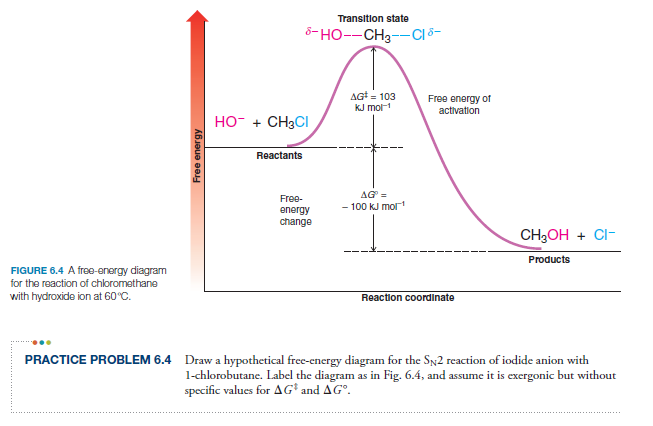
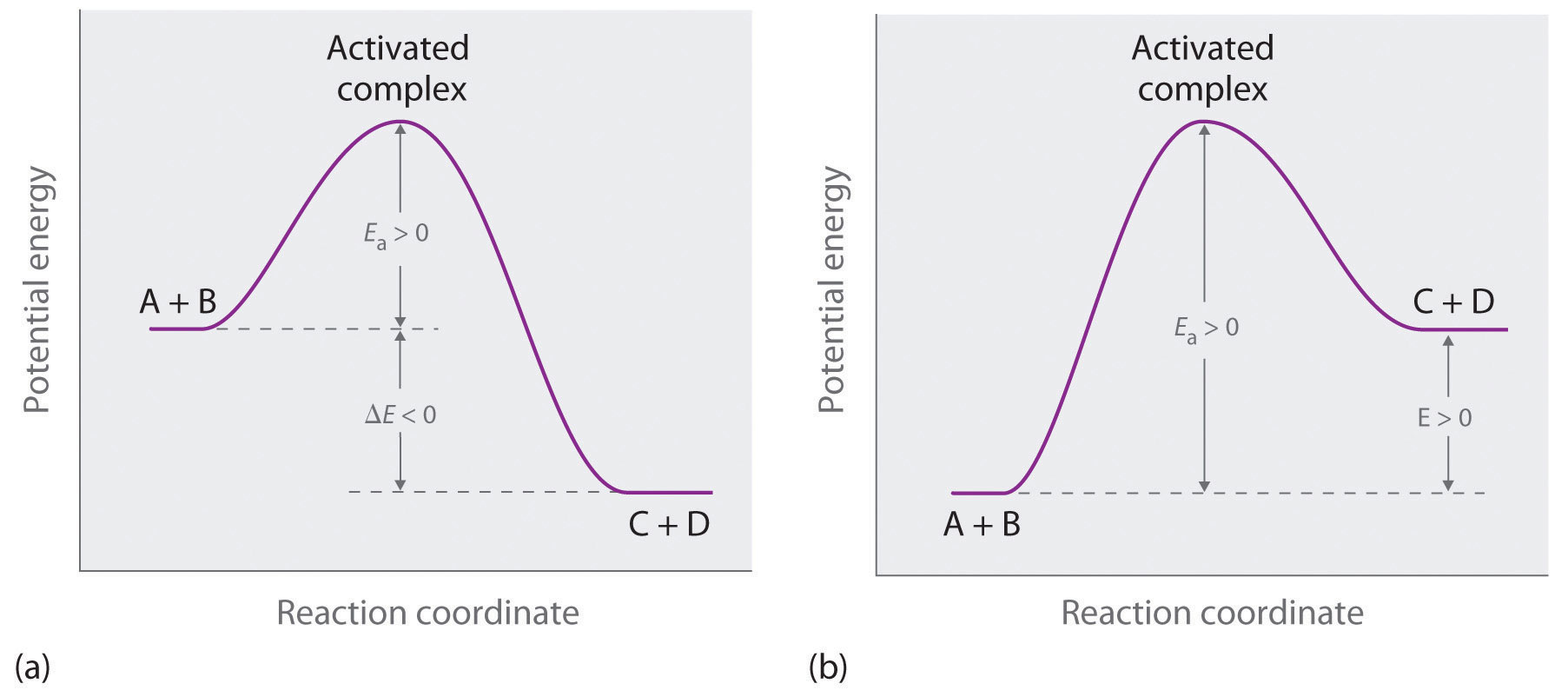
Activation energy vs. delta G - CHEMISTRY COMMUNITY Activation energy vs. delta G. For a reaction profile with potential energy as the Y-axis, the difference between the reactants and the transition state is the activation energy. I thought that if a reaction profile has Delta G for the Y-axis, then the difference between reactants and products will be the delta G with the 2 pluses.
PDF Free Energy Diagram to Phase Diagram Example MS15a, Gibbs Free Energy and Phase Diagrams 11/00 . The system can, in fact, lower its free energy even further by splitting up into a solid of composition X. S B. and a liquid of composition X. L B (shown on both diagrams). The gibbs free energy of the solid is given by point (4) on the g(X. B) diagram and that of the liquid by point (5) on ...
Free Energy and Equilibria - University of Texas at Austin For example this diagram shows a reaction for which \ (\Delta G_ {\rm r}^ {\circ}\) is negative so it "favors" the products. This is a plot of the Gibbs free energy (labeled as the Gibbs Function) versus the extent of the reaction. When the extent of the reaction is zero, the mixture is 100% reactant molecules.
Energy Balances — Introduction to Chemical and ... Exercise: Energy balance for a system with a chemical reaction. Suppose the following reaction is carried out in a chemical reactor: \(\ce{A + B -> C}\). The reactor has a single inlet and a single effluent (outlet) and the entire reactor system is at constant density (\(\rho = \SI{0.9}{kg/L}\)).The desired conversion of \(A\) is \(0.8\).. Operating conditions and parameter values
PDF Energy Diagrams I - Kansas State University B-6. Sketch the potential energy diagram of the car by subtracting it from the kinetic energy diagram. To maintain conservation of energy the potential energy must be negative in the region near the magnet. In fact, the shapes of the potential and kinetic energy diagrams turned out to be identical, although inverted.
Endothermic Reaction | Characteristics, Examples ... Both spontaneous and non-spontaneous endothermic reactions see an increase in the free energy of the System {eq} ( \Delta G > 0 ) {/eq} What are Energy Level Diagrams? The relative energy values of...
Block on an Inclined Plane | Work, Energy and Power Step 2: Work done by non-conservative force during round trip. The work done by the non-conservative force we will call \(W_F\).. We have not been given a magnitude or direction for \(\vec{F}\), all we do know is that it must result in the block moving up the slope.. We have represented the non-conservative force on the force diagram with an arbitrary vector.
Making sense of ∆G and ∆G°, when it comes to equilibrium ... The deviation of delta G from delta G0 is given by: delta G = delta G0 + RTlnQ, where Q = product/reactants expression. When Equilibrium is obtained, delta G = 0, So delta G0 = -RTln K, ie. Q is now K as Q was for non equilibrium. So delta G0 is not necessarily 0 be it at 25 deg C and 1 atmosphere or otherwise. Delta G = 0 at equilibrium, not ...
Relationship Between Electrode Potential, Gibb's Free ... Here, \({\rm{\Delta G}}\) is the difference in the energy between the reactants and the products. \({\rm{\Delta G}}\) is unaffected by external factors such as a change in temperature or the presence of a catalyst that changes the kinetics of the reaction.
Gibbs Free Energy - Purdue University The Gibbs free energy of a system at any moment in time is defined as the enthalpy of the system minus the product of the temperature times the entropy of the system. G = H - TS. The Gibbs free energy of the system is a state function because it is defined in terms of thermodynamic properties that are state functions.
Lecture 8. L. Chasin In an energy diagram: Note that the Delta G is independent of the route between the starting reactants and the final products (say, 3 kcal/mole, for all 4 routes shown here). Free energy is that part of the energy change associated with a chemical reaction that can be harnessed to perform work. We can measure Delta G according to the following ...
Jablonski diagram - Wikipedia In molecular spectroscopy, a Jablonski diagram is a diagram that illustrates the electronic states of a molecule and the transitions between them. The states are arranged vertically by energy and grouped horizontally by spin multiplicity. Nonradiative transitions are indicated by squiggly arrows and radiative transitions by straight arrows. The vibrational ground states of each electronic ...
Dirac Delta Function - an overview | ScienceDirect Topics Based on the concept of Dirac delta function, some important properties of the fundamental solution G ∗ (x, x s) for the linear operator L can be obtained as follows: • G ∗ (x, x s) is defined everywhere, except at x = x s, where it is singular. • L ∗ G ∗ (x, x s) = 0 for the case at x ≠ x s. • The reciprocity relation G ∗ (x ...
Easily Understand IOT Block Diagram and ... - ETechnoG You can easily understand the whole working concept of IoT or Internet of Things by observing the above block diagram carefully. Also, you can see there is a total of six main parts of the IoT. Now let's discuss each block of the diagram.
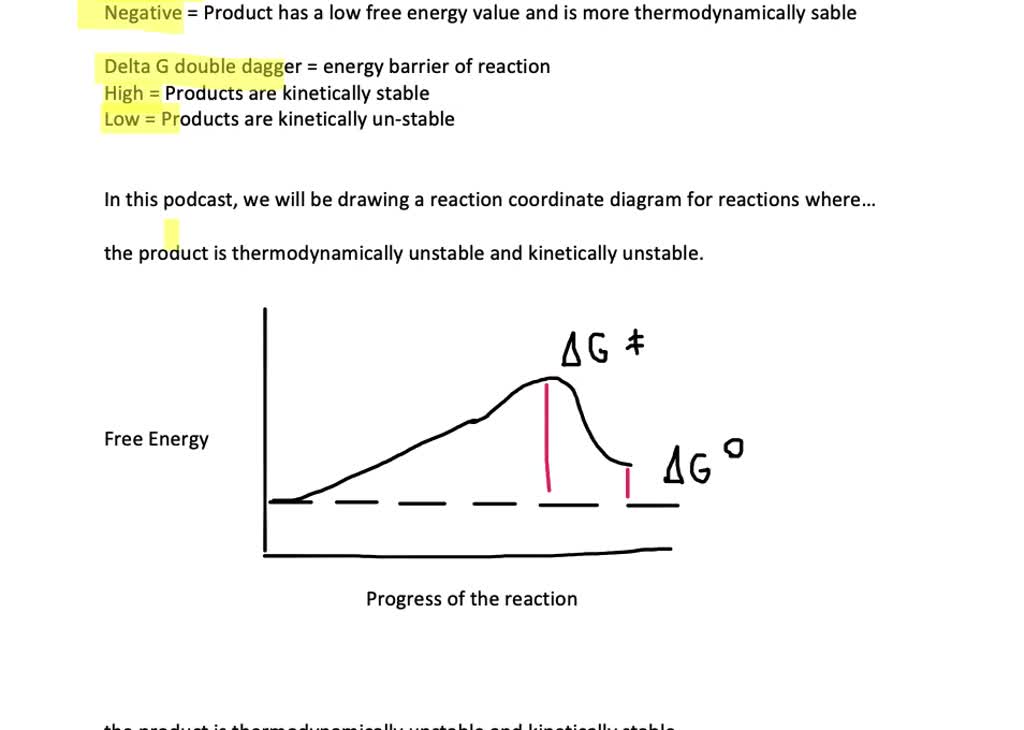
draw a reaction coordinate diagram for a reaction in which a the product is thermodynamically unst 2
Gibbs Free Energy D G o (a delta G, with a superscript o), is the free energy change for a reaction, with everything in the standard states (gases at 1 bar, and solutions at 1 M concentration), and at a specific temperature (usually 25°C) D G (just delta G). This is the free energy change for a reaction that is not at the standard state.
Gibbs free energy - Wikipedia In thermodynamics, the Gibbs free energy (or Gibbs energy) is a thermodynamic potential that can be used to calculate the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure. The Gibbs free energy (. Δ G = Δ H − T Δ S {\displaystyle \Delta G=\Delta H-T\Delta S}
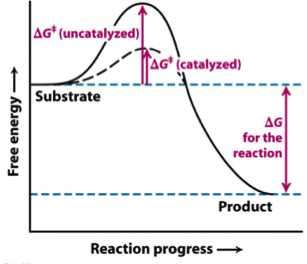
Solved The Gibbs free energy of activation (ΔG+)(delta G ... The Gibbs free energy of activation (ΔG+)(delta G dagger) is Gibbs free energy diagram.png the difference between the uncatalyzed transition state and the products the difference between the substrate/reactants and the transition state the difference between the substrate/reactants and products the difference between the catalyzed transition state and the products the difference between the ...
SOLVED:Consider the following diagram of free energy (G ... We have a diagram of the Gibbs Free Energy. Let's use the information can determine the sign of Delta Jeannot and the equilibrium Constant. So we're told that looking at this minimum free energy is reached when approximately 0.33 of be has been changed to be at this point, the pressure of a is 0.33 times the original pressure.
Internal Energy Questions and Answers | Study.com Find Delta E for the reaction below if the process is carried out at a constant pressure of 1.00 atm and the volume change is -24.5 L. 2CO(g) + O2(g) arrow 2CO2(g); Delta H = -566 kJ View Answer
Ellingham diagram represents A Change of Delta G with ... The Ellingham diagram is a curve related to the value of the Gibbs free energy with temperature. Gibbs energy change is, $\Delta G=\Delta H-T\Delta S$ ---- (1) Where $\Delta H$ are the change in enthalpy and $\Delta S$ the change in entropy. From the above reaction, Gibbs free energy related to the equilibrium constant as,
Gibbs Free Energy | ΔG = ΔH - TΔS - Chad's Prep® Finally, the change in Gibbs free energy is zero (ΔG=0) for a reaction that has reached equilibrium. These are summarized in the table below. ΔG = ΔH - TΔS The change in Gibbs free energy (ΔG) for a system depends upon the change in enthalpy (ΔH) and the change in entropy (ΔS) according to the following equation: ΔG = ΔH - TΔS ΔGo = ΔHo - TΔSo
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...













0 Response to "36 delta g energy diagram"
Post a Comment