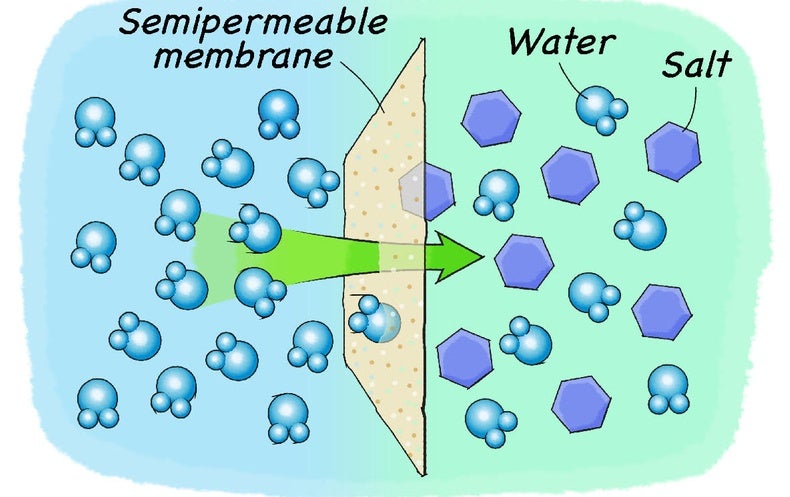
36 salt dissolving in water diagram
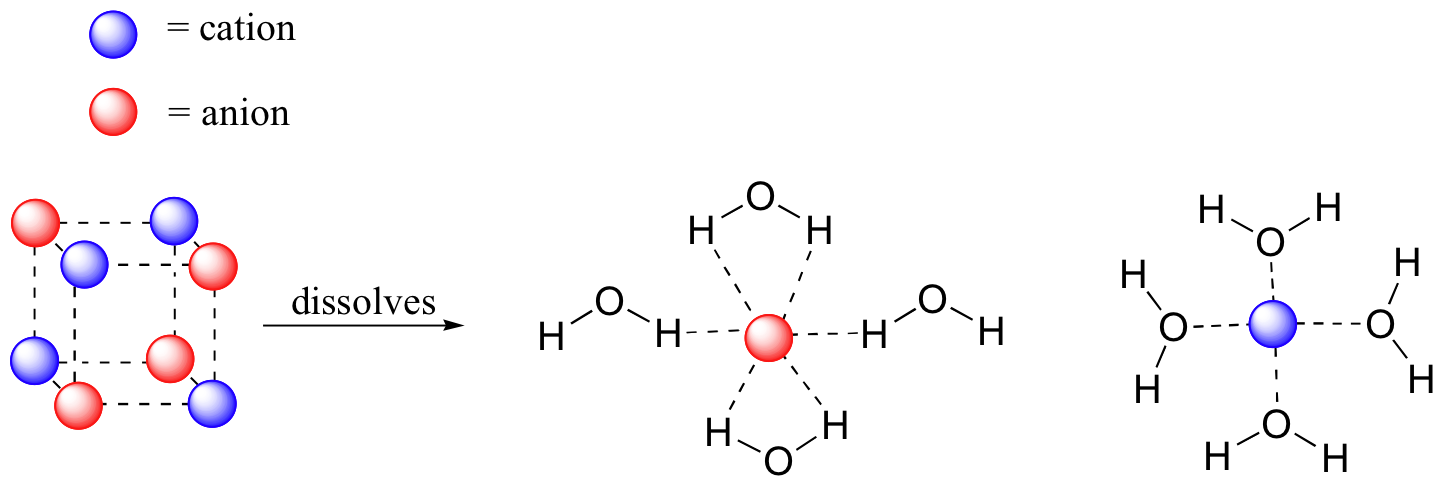
Dissolving Salt in Water - msnucleus.org Students should measure about 100 ml of warm water (with beaker) and mix with 5 ml of Epsom salt (with spoon) into the water. They will pour half of the solution in another jar and place one end of the cotton string (mop string) in each of the two jars with Epsom salt solution, as shown in the diagram on the right. Dissolution of NaCl in Water - interactive simulations ... If you mix two substances and the result is a homogeneous mixture, you are dealing with a solution. In the case of table salt mixed with water, Na and Cl atoms, initially bonded together in the form of a crystal, are dissolved by molecules of water. Water is a solvent. The reasons are electrostatic in nature. The cohesion of atoms and molecules derive from electrostatic links between particles ...
Quick Answer: Is Dissolving Salt In Water A Physical ... What is the process of dissolving salt in water? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows.

Salt dissolving in water diagram
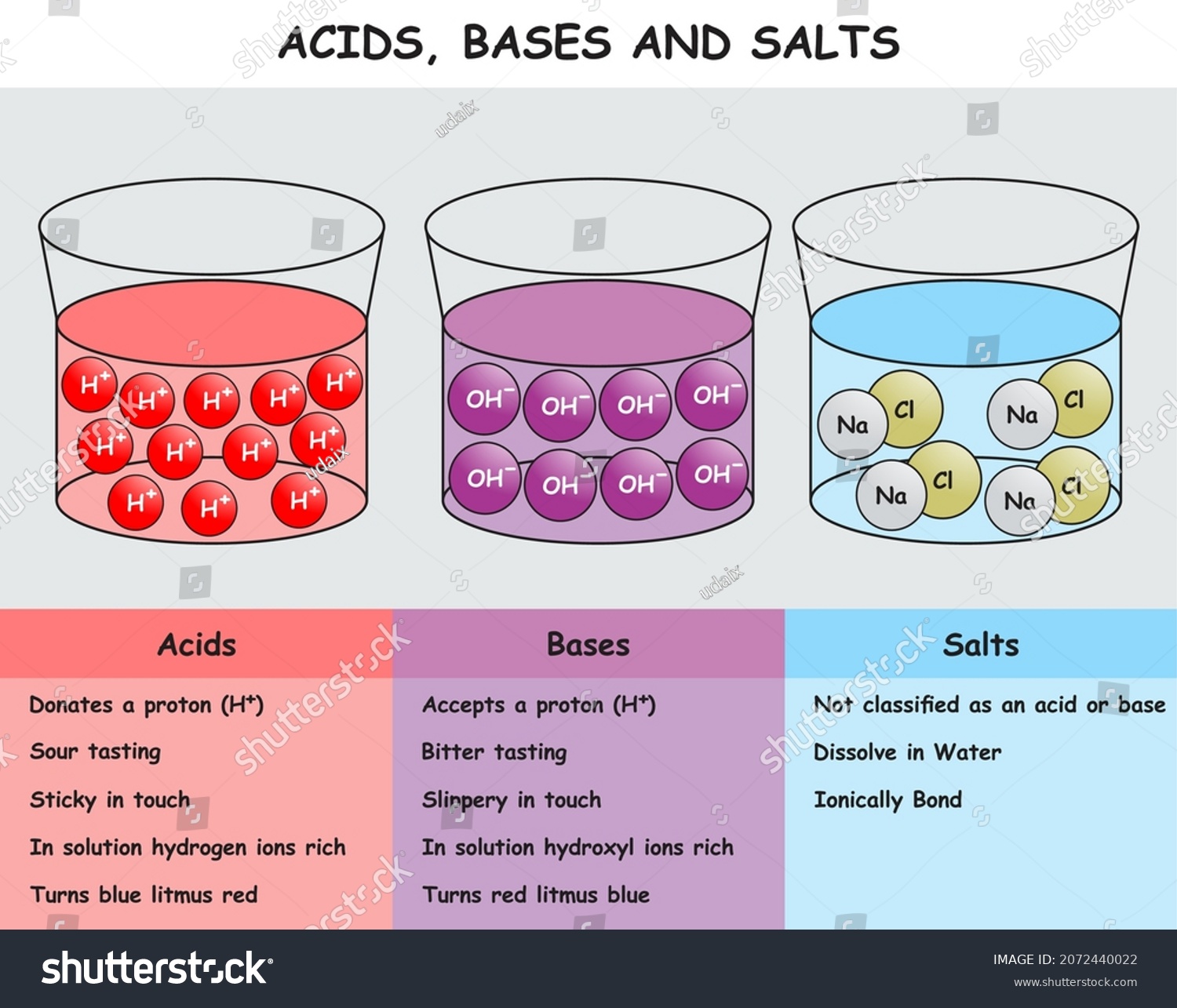
Dissolving Salt in Water: Chemical or Physical Change? When you dissolve salt in water, the sodium chloride dissociates in Na + ions and Cl - ions, which may be written as a chemical equation : NaCl (s) → Na + (aq) + Cl - (aq) Therefore, dissolving salt in water is a chemical change. The reactant (sodium chloride, or NaCl) is different from the products (sodium cation and chlorine anion). PDF Chapter 5, Lesson 3—Why Does Water Dissolve Salt? In separate cups, measure two samples of salt that weigh 5 g each. 2. Place 15 mL of water and alcohol into separate cups. 3. At the same time, add the water and alcohol to the samples of salt. 4. Swirl both cups the same way for about 20 seconds and check for the amount of salt dissolved. 5. Swirl for another 20 seconds and check. Solved A) Draw two molecules of MgCl2 dissolved in water ... Draw two molecules of MgCl2 dissolved in water (show at least 6 water molecules and their interactions with each other and with the salt ions). (HINT: MgCl2 is a salt and made with ionic bonds). In your diagram, label at least one hydrogen and one polar-covalent bond.
Salt dissolving in water diagram. What Happens When Salt Is Added to Water? - Sciencing Solids dissolving in a liquid solvent (usually water in chemistry experiments) create a solution, and salt dissolving in water is a classic example of how a polar solute behaves in a polar solvent such as H 2 O. Along the way, you'll get a side dish of acid-base chemistry just to round out the "flavor" of the salt-water experience! How to Dissolve Salt in Water: 9 Steps (with Pictures ... Choose a salt. There are many different salts available, and they all have different properties.The amount of Epsom salt (MgSO 4) that you can dissolve into a given amount of water at a given temperature will differ from the amount of table salt (NaCl) that you can dissolve into the same water.. If you are trying to understand the dissolution process in general, you should stick with using ... How Does Salt Dissolve in Water? - Reference.com Water dissolves salt by dissociating the ions in salt from each other. Because water is a polar molecule, each of its ends holds a slight positive or negative electrical charge. These ends attract the positive and negative ions in salt and pull them apart from each other. The polarity of water comes from the differences in electronegativity in ... How Water Dissolves Salt - YouTube Water molecules pulling apart the ions (sodium and chloride) in a salt crystal, and then dissolving the salt. ... Water molecules pulling apart the ions (sodium and chloride) in a salt crystal ...
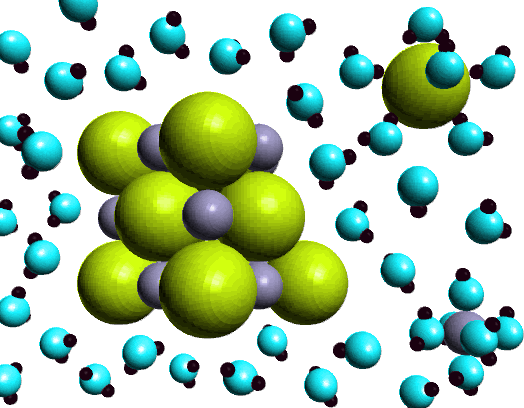
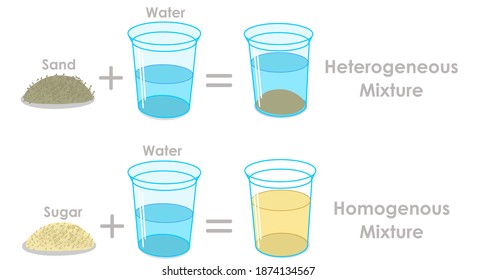
SOLUBILITY Diagram | Quizlet Salt dissolves in water. So you would say, "Salt is _____ in water. SOLUBLE. Sand doesn't dissolve in water so you would say" Sand is _____ in water. INSOLUBLE. soluble. able to be dissolved. Insoluble. a substance that cannot be dissolved in a given solvent. Cocoa mix is an example of a _____ How does sodium chloride (NaCl) dissolve in water? Sodium chloride (NaCl) dissolves when water molecules continuously attack the NaCl crystal, pulling away the individual sodium (Na +) and chloride (Cl -) ions. This nonstop attack continuous until the whole NaCl crystal disintegrates. To understand this process at the molecular level, we must apply the three steps we previously discussed. what happens when nacl is dissolved in water - Lisbdnet.com Explanation: Dissolving sodium chloride in water is a chemical change forming a solution of sodium and chloride ions. …. The sodium ions will not react violently with the water because the sodium is already oxidized (has lost an electron, forming a cation) as pointed out by anor77. NaCl (s) H2O⇌ Na+ (aq)+Cl− (aq) . Dissolving - Physical changes - KS3 Physics Revision - BBC ... If the water in the solution is evaporated, the solute is left behind. The total mass stays the same during dissolving. For example, if 1 g of salt is dissolved in 100 g of water, the mass of salt...
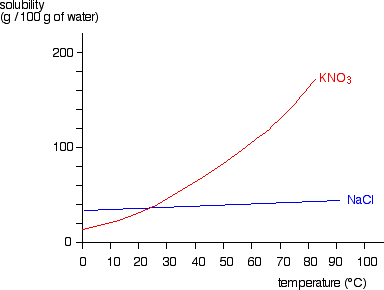
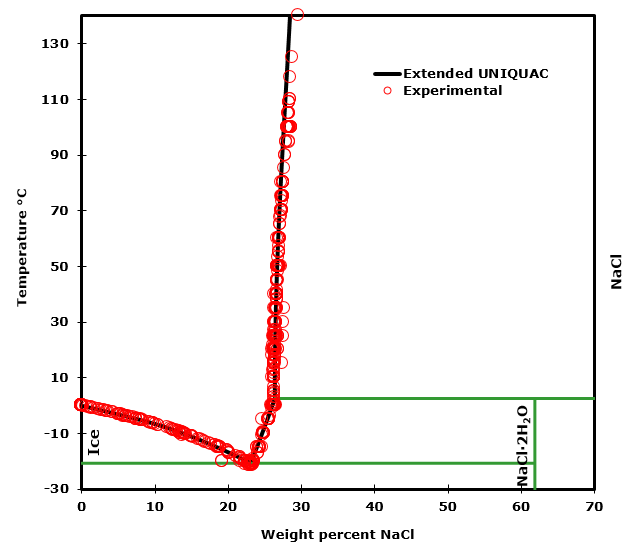
Solvation - Wikipedia Solvation (or dissolution) describes the interaction of solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with solvent, and the strength and nature of this interaction influence many properties of the solute, including solubility, reactivity, and color, as well as influencing the properties of the solvent such as the viscosity and density. Solid-liquid Phase Diagrams: Salt Solution We'll take a solution containing 100 g of potassium nitrate and 100 g of water. Now let the solution cool. At all temperatures above that marked on the graph (about 57°C), 100 g of water will dissolve more than 100 g of potassium nitrate. All the potassium nitrate will stay in solution. At 57°C, you hit the solubility curve. Properties of water - Wikipedia Water has a very high specific heat capacity of 4184 J/(kg·K) at 25 °C – the second-highest among all the heteroatomic species (after ammonia), as well as a high heat of vaporization (40.65 kJ/mol or 2257 kJ/kg at the normal boiling point), both of which are a result of the extensive hydrogen bonding between its molecules. These two unusual properties allow water to … Draw a diagram of table salt (NaCl) dissolved in water ... Find step-by-step Biology solutions and your answer to the following textbook question: Draw a diagram of table salt (NaCl) dissolved in water..
Why Does Water Dissolve Salt? | Chapter 5: The Water ... In separate cups, measure two samples of salt that weigh 5 g each. Place 15 mL of water and alcohol into separate cups. At the same time, add the water and alcohol to the samples of salt. Swirl both cups the same way for about 20 seconds and check for the amount of salt dissolved. Swirl for another 20 seconds and check.
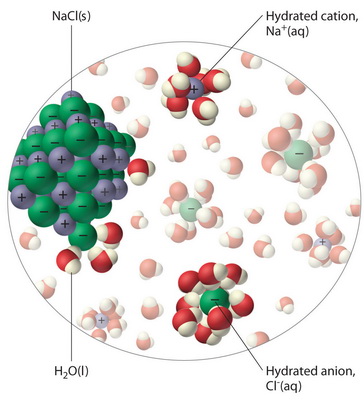
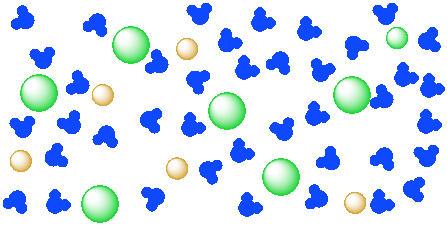
Water, the Universal Solvent | U.S. Geological Survey Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
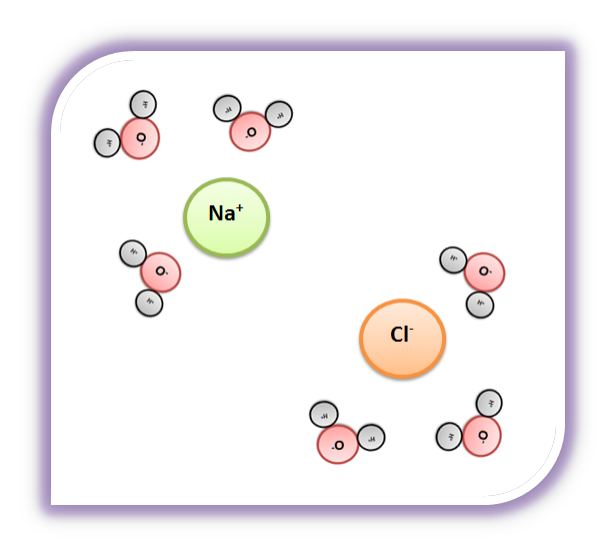
⚗️The diagram shows salt dissolved in water. What does it ... The diagram shows salt dissolved in water. What does it show about water molecules - 20635482 simonenekia2000 simonenekia2000 01/14/2021 Chemistry College answered The diagram shows salt dissolved in water. What does it show about water molecules and chloride ions? + Chloride Oxygen Hydrogen 4 Sodium 1
Salt vs. Sugar - A Dissolving Problem | Chemical Education ... When a substance dissolves in water, and each water molecule is like a tiny magnet. For a substance to dissolve in water, it must also be a polar molecule, or it must be capable of breaking into polar molecules. For example, when you add some salt in water it can be dissolved into water and become salt water. No, dissolve differently.
DIAGRAM >> A representation of the dissolving of sodium ... DIAGRAM >> A representation of the dissolving of sodium molecules in water. The negative ends of the polar water molecules are attracted to the positive sodium ions, and pull them off the crystal lattice. The positive ends of the water molecules are attracted to the negative chloride ions, and pull them off.
How does water dissolve salts - Florida State College at ... These free ions in a salt-water solution allow electricity to flow through water. Ionic compounds such as sodium chloride, that dissolve in water and dissociate to form ions, are called electrolytes. Please Watch animation 10.3 on ionic solutions.
SOLVED:Draw a diagram of table salt (NaCl) dissolved in water. Video Transcript. we can easily draw a diagram of sodium chloride dissolved in water. Let's just do some quick review, though. Sodium chloride is in a C l. And remember, sodium has a positive charge because it has one extra proton in the nucleus than electrons in the outer cloud. And chloride has one extra electrons in the outer plowed compared ...
What dissolves in salt ionic? - JacAnswers What dissolves in salt ionic? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
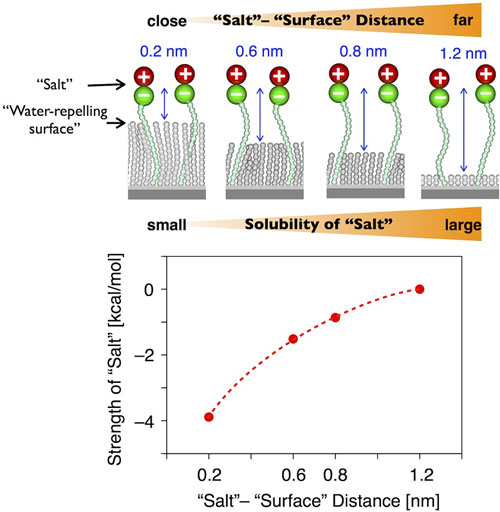
Why Is Dissolving Salt In Water Exothermic ... 10. Sketch the Energy Diagram (from our notes) for each of the dissolving processes. Why is dissolving Epsom salt into water endothermic? An endothermic reaction is a chemical reaction that requires heat in order to occur. … For this experiment, the Epsom salt is absorbing the heat from the water, thus causing the temperature of the water to ...
44 diagram of salt dissolved in water - Wiring Diagram Source Diagram of salt dissolved in water Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
What happens when sodium chloride NaCl is dissolved in water? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
Water molecules and their interaction with salt | U.S ... Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution. Find out more Adhesion/cohesion
Solubility Diagram - ScienceGeek.net Solubility Diagram. Show all questions. 1 / 12. At approximately what temperature does the solubility of sodium chloride, NaCl, match the solubility of potassium dichromate, K 2 Cr 2 O 7? 83 ºC. 60 ºC. 50 ºC. 30 ºC. Which salt is LEAST soluble at 0 ºC?
FAQ: What kind of change occurs when salt dissolves in water? What happens when salt dissolves in water? Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
(PDF) GRADE 6 SCIENCE ACTIVITIES FIRST ... - Academia.edu Mixtures Predictions Observation Explain Salt in water Coffee in water Mud in water 2. After giving your predictions, add the materials to the water. Then, write your observations. 3. For every observation, give a reasonable explanation. Guide Questions: 1. What are the states of the materials used that were combined with water? 2. What happened to these materials when …
Solved A) Draw two molecules of MgCl2 dissolved in water ... Draw two molecules of MgCl2 dissolved in water (show at least 6 water molecules and their interactions with each other and with the salt ions). (HINT: MgCl2 is a salt and made with ionic bonds). In your diagram, label at least one hydrogen and one polar-covalent bond.
PDF Chapter 5, Lesson 3—Why Does Water Dissolve Salt? In separate cups, measure two samples of salt that weigh 5 g each. 2. Place 15 mL of water and alcohol into separate cups. 3. At the same time, add the water and alcohol to the samples of salt. 4. Swirl both cups the same way for about 20 seconds and check for the amount of salt dissolved. 5. Swirl for another 20 seconds and check.
Dissolving Salt in Water: Chemical or Physical Change? When you dissolve salt in water, the sodium chloride dissociates in Na + ions and Cl - ions, which may be written as a chemical equation : NaCl (s) → Na + (aq) + Cl - (aq) Therefore, dissolving salt in water is a chemical change. The reactant (sodium chloride, or NaCl) is different from the products (sodium cation and chlorine anion).

















0 Response to "36 salt dissolving in water diagram"
Post a Comment