36 partial energy level diagram for hydrogen
Grotrian Diagrams - physics.unlv.edu Moore, C. E. & Merrill, P. W. 1968, Partial Grotrian Diagrams of Astrophysical Interest, 1968 (Washington, D.C.: GPO) A great collection of well-drawn figures that have been of great use over the decades---but it only has selection of most useful Grotrian diagrams and some of the data may be out of date or incomplete. How To Draw Energy Level Diagrams - YouTube In this video, I will teach you how to draw energy level diagrams, which is a better way of visualizing how electrons are laid out within an atom. It also p...
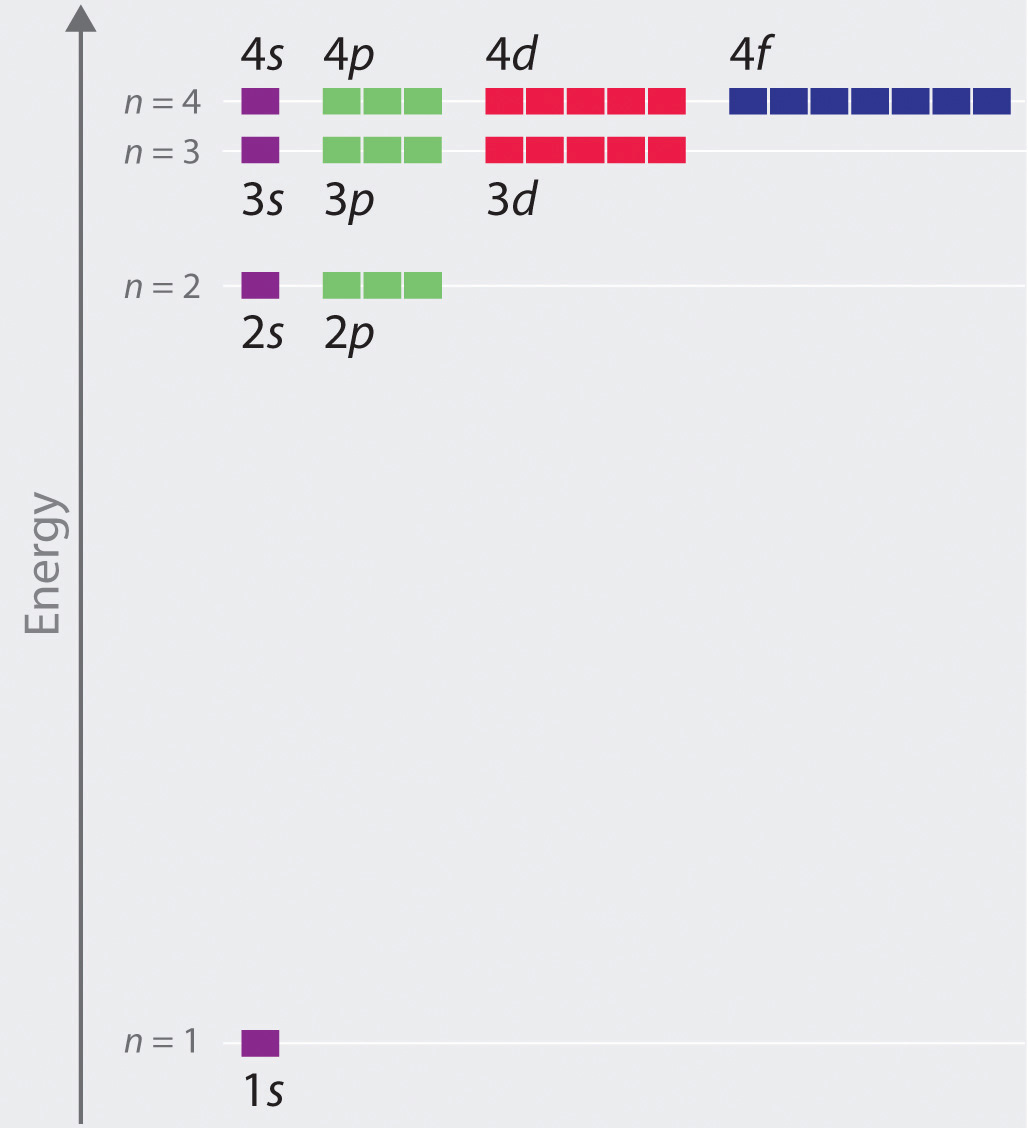
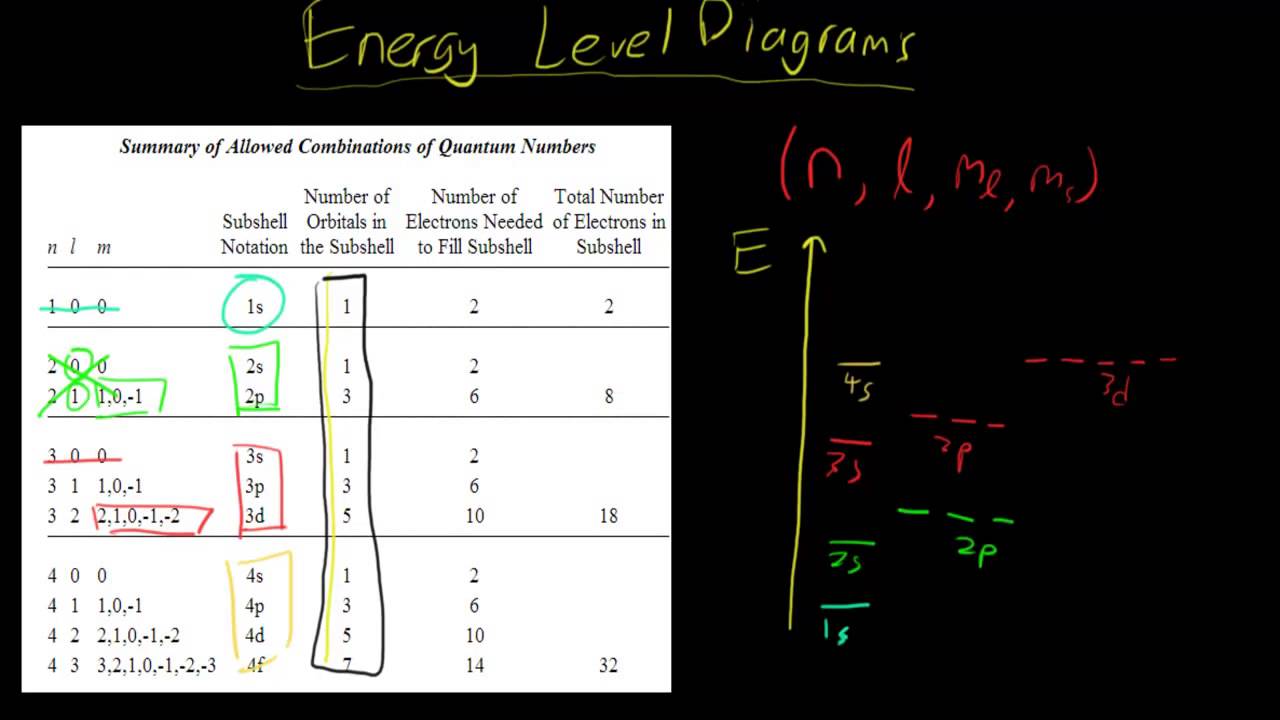
Energy Level Diagram - Different Energy Shells Around the ... Below is a blank energy level diagram which helps you depict electrons for any specific atom. At energy level 2, there are both s and p orbitals. The 2s has lower energy when compared to 2p. The three dashes in 2p subshells represent the same energy. 4s has lower energy when compared to 3d. Therefore, the order of energy level is as follows:
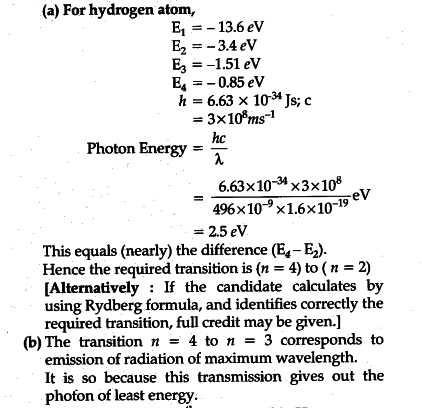
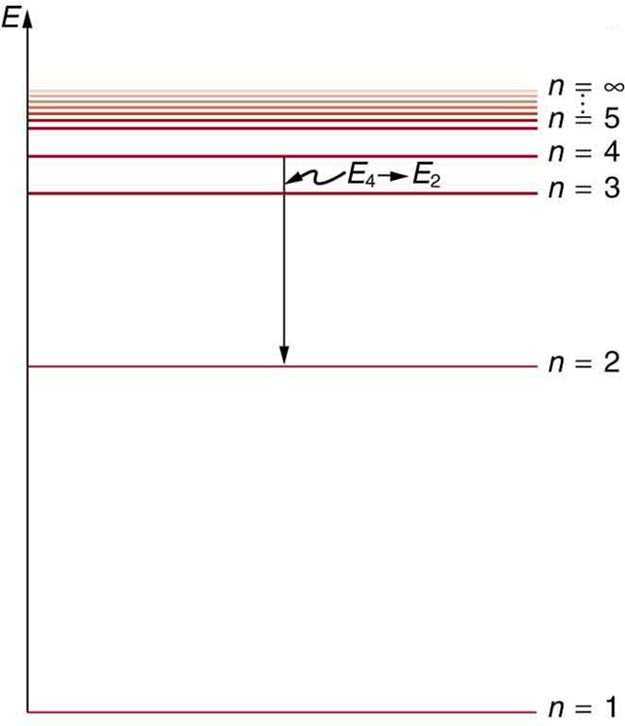
Partial energy level diagram for hydrogen
Emission spectrum of hydrogen (video) - Khan Academy So, those are electrons falling from the higher energy levels down to the 2nd energy level. So, let's go ahead and draw them on our diagram here. So, let's say an electron fell from the 4th energy level down to the 2nd. Alright, so, that energy difference, if you do the calculations, that turns out to be the blue-green line in your line spectrum. Hydrogen(H) electron configuration and orbital diagram Hydrogen (H) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction. This is clearly shown in the figure of the orbital diagram of hydrogen. Atomic Orbital Diagram for Hydrogen (H) Partial Energy Level Diagram for Hydrogen | Physics Forums Related Threads on Partial Energy Level Diagram for Hydrogen Energy level diagram of a chemical reaction. Last Post; Jul 30, 2020; Replies 4 Views 781. Energy in Liquid Hydrogen. Last Post; Apr 30, 2010; Replies 1 Views 2K. Energy state of hydrogen molecule. Last Post; Aug 1, 2012; Replies 1 Views 3K. P. Hydrogen bonds and binding energy ...
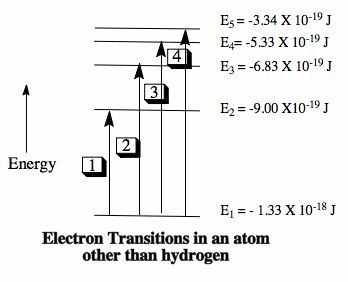
Partial energy level diagram for hydrogen. CHEM MIDTERM #1 (Hwk Questions) Flashcards | Quizlet Consider the following portion of the energy-level diagram for hydrogen: n = 4-0.1361 × 10-18 J n = 3-0.2420 × 10-18 J n = 2-0.5445 × 10-18 J n = 1-2.178 × 10-18 J For which of the following transitions does the light emitted have the longest wavelength? L23.1 Energy levels and diagram for hydrogen. - YouTube MIT 8.04 Quantum Physics I, Spring 2016View the complete course: : Barton ZwiebachLicense: Creative Commons BY-NC-SAMore ... The following question relates to the partial energy level ... The following question relates to the partial energy level diagram for the hydrogen electron: .54eV n5 .85eV n4 1.51eV n3 3.4eV n2 13.6eV n1 The question relates to electron located at E3 . What is the emission energy when the electron falls to E1 from E3 ? PDF The Decomposition of Hydrogen Peroxide - Chem21Labs level diagram, or potential energy profile, as shown in Figure 13.1. The vertical axis gives the potential energy for the reaction, while the horizontal axis is a relative (i.e., time) scale that shows the progress of the reaction. The diagram indicates that there is a "hill" or energy barrier that needs to be overcome before any products ...
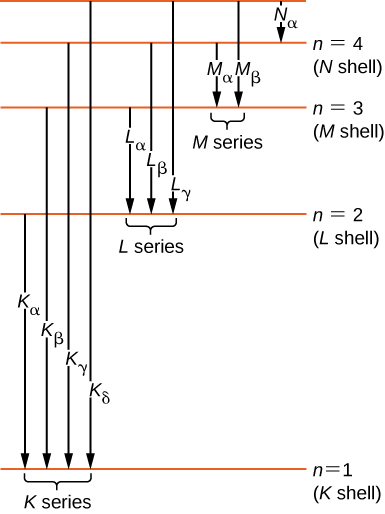
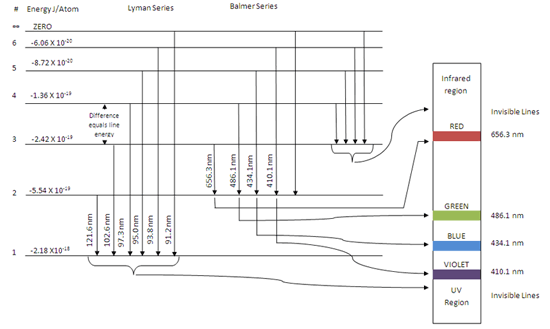
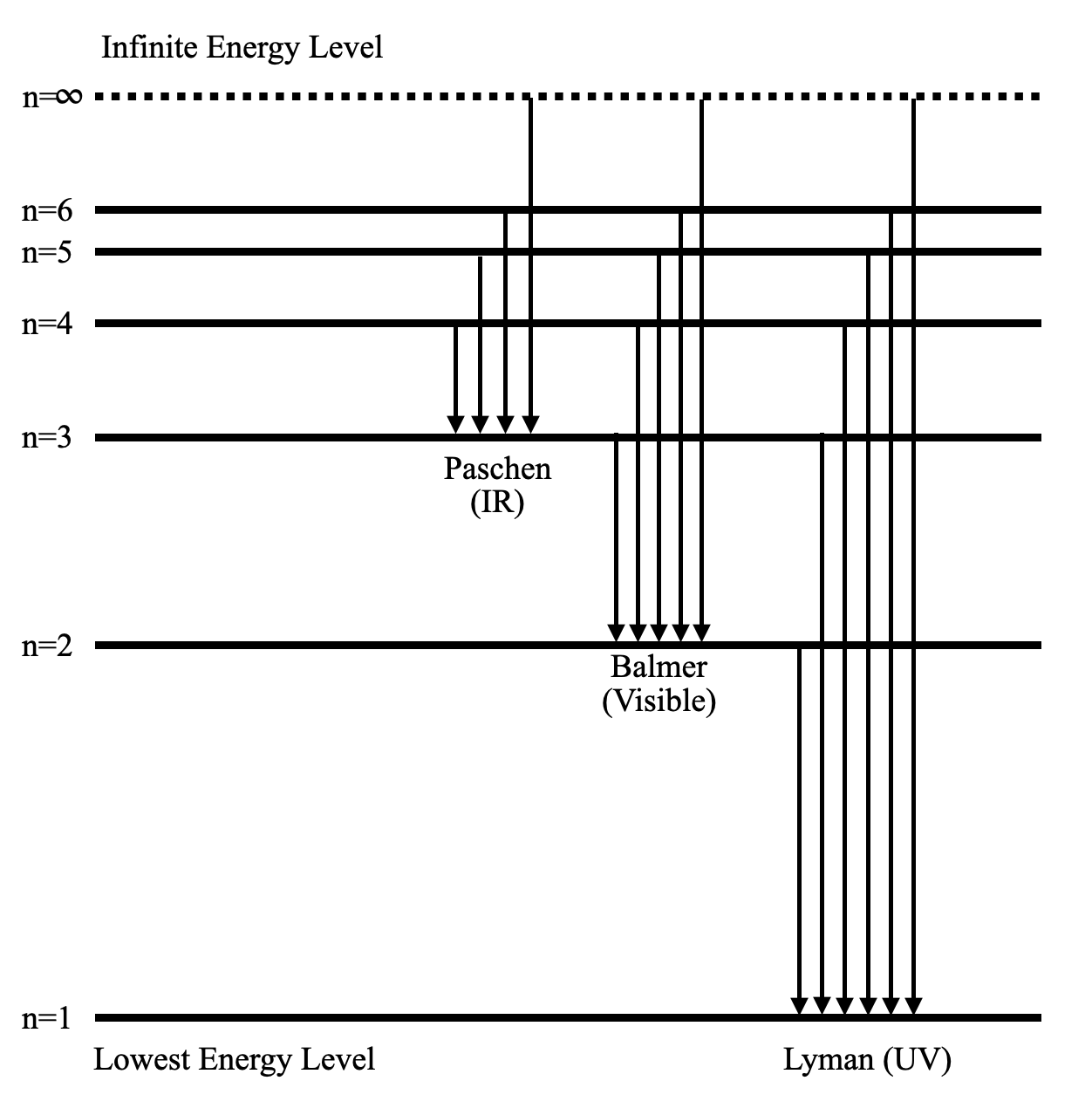
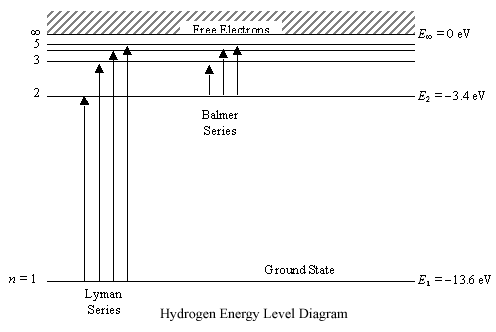
Energy levels of molecular hydrogen. The transitions ... Download scientific diagram | Energy levels of molecular hydrogen. The transitions between the electronically excited energy levels ( B 1 Σ + u and C 1 Π u ) and the electronic ground level ( X ... Energy level diagrams and the hydrogen atom Energy level diagrams and the hydrogen atom It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. PDF IGNITION ENERGY AND IGNITION PROBABILITY ... - Hydrogen Tools IGNITION ENERGY AND IGNITION PROBABILITY OF METHANE-HYDROGEN -AIR MIXTURES Hankinson, G. 1 , Mathurkar, H. 1 and Lowesmith, B.J.1 1 Department of Chemical Engineering, Loughborough University, Leicestershire, LE11 3TU ABSTRACT The European Commission are funding an investigation of the feasibility of using existing natural gas Energy Level Diagram For Hydrogen - Mini Physics The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon.
PDF Energy Level Diagrams - Ms. Scott's Site Energy level diagrams for IONS Atoms with 1, 2, or 3 valence electrons lose them to form 1+, 2+ or 3+ ions respectively. naming metallic ions - the full name of the atom is followed by the word ion. Mg2+ is the magnesium ion Group 1 (1+) (lose 1e) Group 2 (2+) (lose 2e) Group 13 (3+) (lose 3e) Energy level diagrams for IONS How do yo write the orbital diagram for hydrogen? | Socratic See below. Orbital diagrams are useful to show the number of electrons, number of electron shells, number of electron pairs, and electron spin directions in a particular atom/ion. Arrows represent electrons, and their spin is represented by which way they point (up or down). Two electrons can be paired into one shell (one little box) as one orbital. Groups of boxes right next to each other ... The following question relates to the partial energy level ... The following question relates to the partial energy level diagram for the hydrogen electron: .54eV n5 .85eV n4 1.51eV n3 3.4eV n2 13.6eV n1 The question relates to a hydrogen electron located at E3 .What is the emission energy when the electron falls to E5 from E3 ? CLASSES AND TRENDING CHAPTER class 5 PhysicsLAB: Energy-Level Diagrams Using the Bohr Model, the energy levels (in electron volts, eV) are calculated with the formula: En = -13.6 (Z2/n2) eV where Z is the atomic number and n is the energy level. The ground state is represented by n = 1, first excited state by n = 2, second excited state by n = 3, etc. eV
Draw a partial energy level diagram for hydrogen. All ... Draw a partial energy level diagram for hydrogen. All wavelengths are ending at the n = 2 state and the energy of the n = 2 state is -.545 aJ. Wavelengths(nm): 411.26, 434.77, 487.10, 658.42
Energy Levels of Hydrogen Atom - University of Texas at Austin Energy Levels of Hydrogen Atom. Consider a hydrogen atom, for which the potential takes the specific form. (4.119) The radial eigenfunction satisfies Equation ( 4.118 ), which can be written. (4.120) Here, is the reduced mass, which takes into account the fact that the electron (of mass ) and the proton (of mass ) both orbit about a common ...
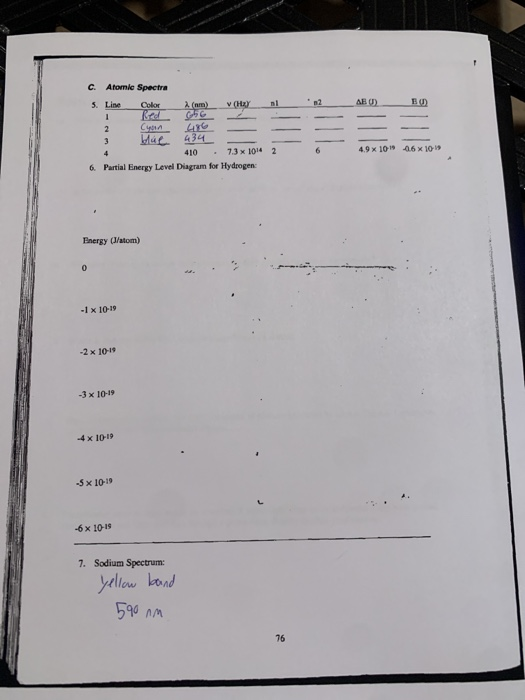
Solved How Do I Make The Partial Energy Level Diagram for ... Line Color 2 (nm) v (Hz) nl AE (J) E (1) 2 4.9 x 10-19 -0.6 x 10-19 410 7.3 x 1014 6. Partial Energy Level Diagram for Hydrogen: Energy (J/atom) -1 x 10-19 -2x 10-19 -3 x 10-19 -4 x 10-19 -5 x 10-19 -6 x 10-19 Previous question Next question
Lithium energy-level diagram - Big Chemical Encyclopedia Lithium energy-level diagram Energy level diagrams for the easily excited atomic lines of lithium, sodium, potassium and rubidium. Wavelengths are given in nanometres for the spectral lines produced by transitions between the different levels. The ionization potential is indicated by the dashed line above the respective diagrams. The Selection Rule for L - The energy-level diagram for lithium ...
E7-OpSpec - Optical Spectroscopy Objective ... - Course Hero For the partial energy-level diagram of hydrogen, assume that all the observed transitions terminate at the n = 2 state; for example, if you observe two transitions, they are from state A → n = 2 and state B → n = 2. Also, set the value of the energy of the n = 2 state to -0.545 aJ.
Partial Energy Level Diagram for Hydrogen - Partial Energy ... Partial Energy Level Diagram for Hydrogen - Partial Energy Level Diagram for Hydrogen 1 = (6.626 x 10-37 KJ) (3.00 x 1017 nm/s) (6.022 x 1023 | Course Hero Partial Energy Level Diagram for Hydrogen - Partial Energy... School Cornell University Course Title CHEM 2070 Type Lab Report Uploaded By ProfessorResolveBadger1305 Pages 1 Ratings 50% (2)
Hydrogen Spectrum Activity - Carolina.com R H is the Rydberg constant (1.097 × 10 7 m-1) for hydrogen, n 1 is the lower-energy level, and n 2 is the higher-energy level. Pre-activity questions. The visible region of the electromagnetic spectrum lies between the wavelengths of _____ and _____ nm. A certain photon has a wavelength of 550 nm. Calculate its energy in Joules.
Hydrogen partial energy-level diagram : HomeworkHelp Then all you need to do is plug your newfound frequency into E = h f and you have your energy level spacings. Note: again look at your units when you look up a value for h. It will probably be most valuable for you if you use the h value that includes electron volts [eV] instead of Joules [J]. I hope this helps.
Partial Energy Level Diagram for Hydrogen | Physics Forums Related Threads on Partial Energy Level Diagram for Hydrogen Energy level diagram of a chemical reaction. Last Post; Jul 30, 2020; Replies 4 Views 781. Energy in Liquid Hydrogen. Last Post; Apr 30, 2010; Replies 1 Views 2K. Energy state of hydrogen molecule. Last Post; Aug 1, 2012; Replies 1 Views 3K. P. Hydrogen bonds and binding energy ...
Hydrogen(H) electron configuration and orbital diagram Hydrogen (H) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction. This is clearly shown in the figure of the orbital diagram of hydrogen. Atomic Orbital Diagram for Hydrogen (H)
Emission spectrum of hydrogen (video) - Khan Academy So, those are electrons falling from the higher energy levels down to the 2nd energy level. So, let's go ahead and draw them on our diagram here. So, let's say an electron fell from the 4th energy level down to the 2nd. Alright, so, that energy difference, if you do the calculations, that turns out to be the blue-green line in your line spectrum.
![Partial energy level diagram [14]. | Download Scientific Diagram](https://www.researchgate.net/profile/Madjid-Diaf/publication/231022369/figure/fig3/AS:667685213974535@1536199968356/Partial-energy-level-diagram-14.png)



















0 Response to "36 partial energy level diagram for hydrogen"
Post a Comment