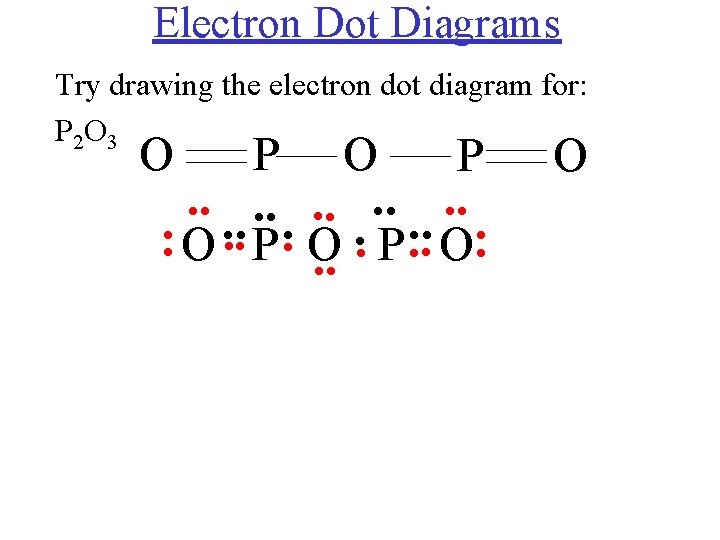
35 lewis electron dot diagram definition
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that ... 22.11.2021 · The SF4 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the SF4 molecule. 062 g/mol, this compound is made up of one Sulfur atom and two Fluoride atoms. For the SF4 structure use the periodic table to find the total n There are a total of 34 valence electrons in the Lewis structure for SF4. There is notable 4 layers of bonding pairs in ...
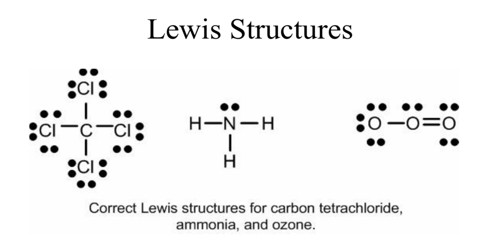
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
Lewis electron dot diagram definition
The lewis diagram of an ionic compound is formed with a different approach than the normal procedure we used for drawing the compounds like NH3, BF3, BrF5, etc. Steps to draw electron dot structure or lewis dot structure of NaCl. Step 1: In the first step, we have to count the valence electron available for drawing the lewis structure of NaCl. For knowing the valence electron you have to ... 24.11.2021 · Definition and Introduction. Lewis Structure is a systematic approach towards deciphering the nature and position of atoms for chemical bonding inside a molecule. This was formulated by Gilbert N Lewis and stands for a diagrammatic representation of bonds and valence electrons of a chemical molecule. Here, we work towards sketching a skeleton diagram of the molecule with atoms … 26.11.2021 · Lewis Structure is one of the key terminologies to understand the chemical bonding of a molecule since it represents the molecular structure. It depends on the octet rule concept and is an extension of the electron dot diagram. Thus, to have a comprehensive idea about CS2 Lewis Structure, let us go through each step clearly and systematically.
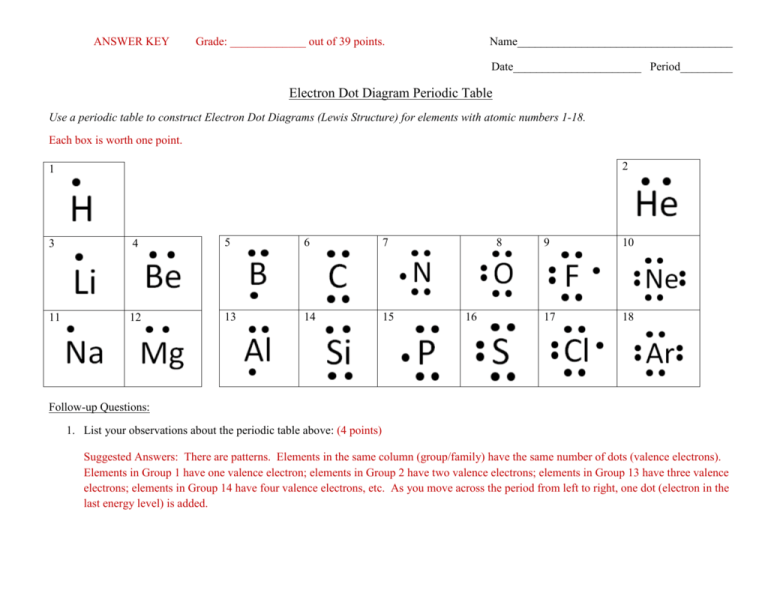
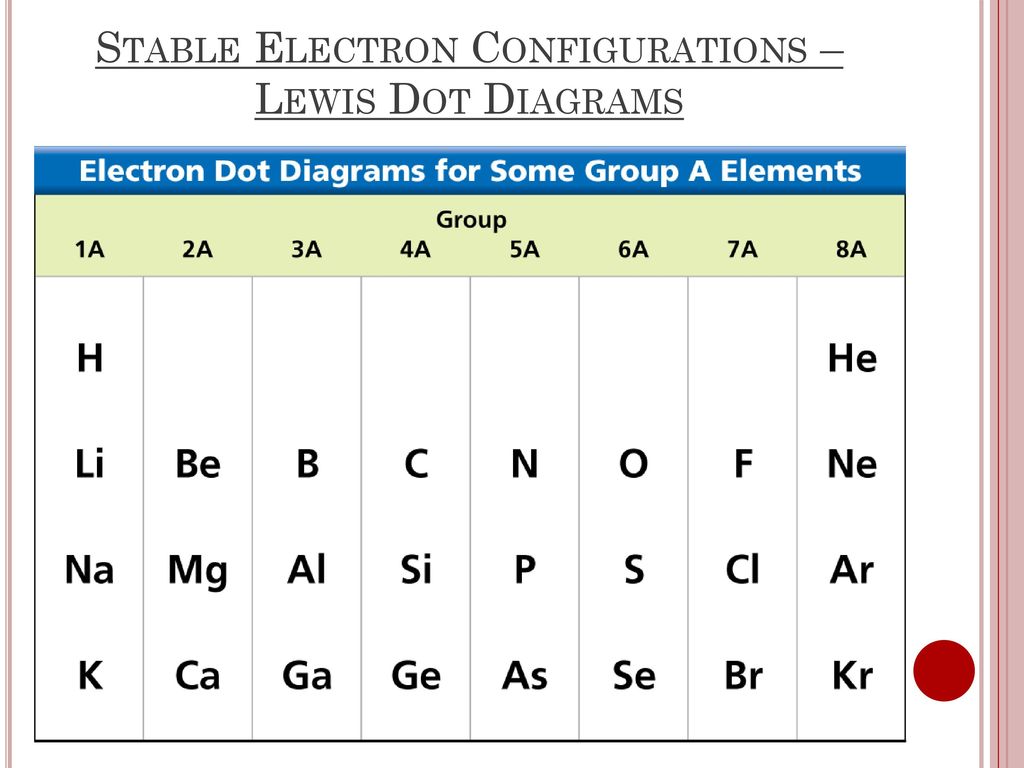
Lewis electron dot diagram definition. Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. 16.10.2019 · Lewis structures go by many names, including Lewis electron dot structures, Lewis dot diagrams, and electron dot structures. All these names refer to the same sort of diagram, which is intended to show the locations of bonds and electron pairs. Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) are diagrams that represent the valence electrons of an atom. A way of representing atoms or molecules by showing electrons as dots surrounding the element symbol. One bond is represented as two ...
Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain ... How to draw Lewis Diagrams. Lewis structure, also called electron-dot structure, is a structural formula in which electrons are represented by dots; two dots between two atoms represent a ... Lewis Electron Dot Diagrams are used to visually depict bonding by representing valence electrons as dots surrounding an elemental symbol. Finally, you'll understand all those weird pictures of molecules with the letters and the lines and the dots! Those are lewis dot structures ...
Review what a Lewis dot diagram is and discover how to draw a Lewis dot structural formula for compounds. Learn how to represent single, double and triple bonds with lines instead of dots. Also ... Lewis acids are diverse. Simplest are those that react directly with the Lewis base. But more common are those that undergo a reaction prior to forming the adduct. Examples of Lewis acids based on the general definition of electron pair acceptor include: the proton (H +) and acidic compounds onium ions, such as NH 4 + and H 3 O + Sf4 2 lewis structure [email protected] 22.09.2021 · The electron arrangement of an atom at its lowest possible energy state is known as the ground state electron configuration. Learn more about the definition of the ground state electron ...
26.11.2021 · Lewis Structure is one of the key terminologies to understand the chemical bonding of a molecule since it represents the molecular structure. It depends on the octet rule concept and is an extension of the electron dot diagram. Thus, to have a comprehensive idea about CS2 Lewis Structure, let us go through each step clearly and systematically.
24.11.2021 · Definition and Introduction. Lewis Structure is a systematic approach towards deciphering the nature and position of atoms for chemical bonding inside a molecule. This was formulated by Gilbert N Lewis and stands for a diagrammatic representation of bonds and valence electrons of a chemical molecule. Here, we work towards sketching a skeleton diagram of the molecule with atoms …
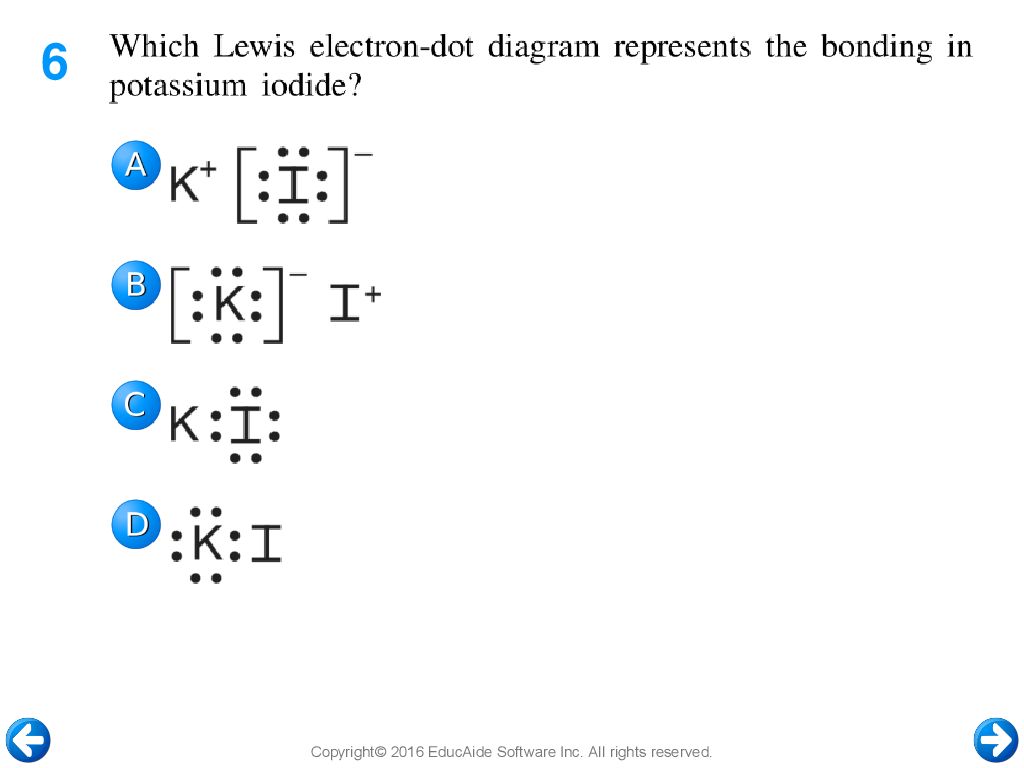
The lewis diagram of an ionic compound is formed with a different approach than the normal procedure we used for drawing the compounds like NH3, BF3, BrF5, etc. Steps to draw electron dot structure or lewis dot structure of NaCl. Step 1: In the first step, we have to count the valence electron available for drawing the lewis structure of NaCl. For knowing the valence electron you have to ...

Unit 3 Atomic Concepts 3 9 What Is A Lewis Diagram Aim How Do I Draw A Lewis Diagram Of Any Element Do Now Draw A Planetary Model Of Oxygen How Many Ppt Download

Use Electron Dots And Or Pairs Of Dots As Appropriate To Show The Lewis Symbol For The Neutral Atom Arsenic Study Com
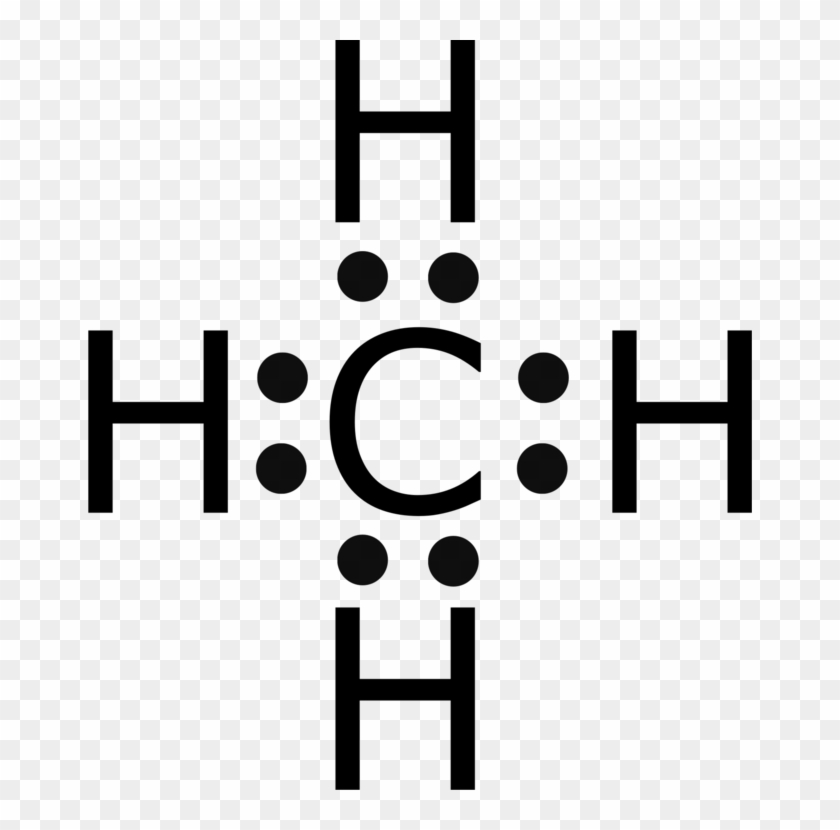
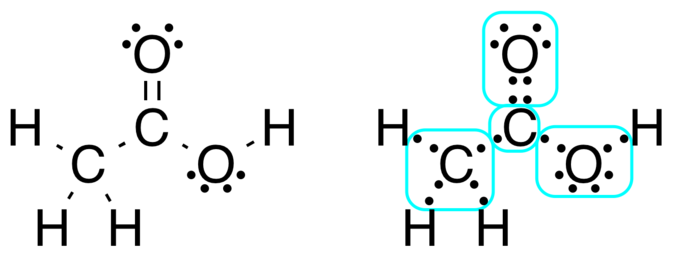
Lewis Structure Methane Electron Atom Hydrogen Lewis Dot Diagram Of Methane Free Transparent Png Clipart Images Download













/lewisnitrite-56a128825f9b58b7d0bc90cf.jpg)
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)

:max_bytes(150000):strip_icc()/ICl3_LD-56a12a2b3df78cf77268034c.png)


0 Response to "35 lewis electron dot diagram definition"
Post a Comment